| Identification |
|---|
| YMDB ID | YMDB00140 |
|---|
| Name | 6-Phosphonoglucono-D-lactone |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Baker's yeast |
|---|
| Description | 6-Phosphonoglucono-D-lactone, also known as D-glucono-1,5-lactone 6-phosphate or 6-PGDL, belongs to the class of organic compounds known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. 6-Phosphonoglucono-D-lactone is an extremely weak basic (essentially neutral) compound (based on its pKa). 6-Phosphonoglucono-D-lactone exists in all living species, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | - 3alpha,7alpha,12alpha-trihydroxy-24-oxo-5beta-cholestan-26-oyl-coenzyme A
- 3alpha,7alpha,12alpha-Trihydroxy-5beta-24-oxocholestanoyl-CoA
- 6-(dihydrogen phosphate)-(8CI)-D-Gluconic acid delta-lactone
- 6-(dihydrogen phosphate)-(9CI)-D-Gluconic acid delta-lactone
- 6-(dihydrogen phosphate)-D-Gluconic acid delta-lactone
- 6-Pgdl
- 6-Phospho-D-glucono-1,5-lactone
- 6-Phosphoglucono-delta-lactone
- 6-phosphogluconolactone
- 6-Phosphonoglucono-delta-lactone
- D-6-phospho-glucono-delta-lactone
- D-6-phosphoglucono-delta-lactone
- D-Glucono-1,5-lactone 6-phosphate
- D-glucono-delta-lactone-6-phosphate
- delta-Gluconolactone 6-phosphate
- [(2R,3S,4S,5R)-3,4,5-Trihydroxy-6-oxotetrahydro-2H-pyran-2-yl]methyl dihydrogen phosphate
- D-Gluconic acid, delta-lactone, 6-(dihydrogen phosphate)
- 6-Phosphonoglucono-δ-lactone
- [(2R,3S,4S,5R)-3,4,5-Trihydroxy-6-oxotetrahydro-2H-pyran-2-yl]methyl dihydrogen phosphoric acid
- D-Gluconate, delta-lactone, 6-(dihydrogen phosphate)
- D-Gluconate, δ-lactone, 6-(dihydrogen phosphate)
- D-Gluconic acid, delta-lactone, 6-(dihydrogen phosphoric acid)
- D-Gluconic acid, δ-lactone, 6-(dihydrogen phosphoric acid)
- D-Glucono-1,5-lactone 6-phosphoric acid
- p-Gluconolactone
|
|---|
| CAS number | 2641-81-8 |
|---|
| Weight | Average: 258.1199
Monoisotopic: 258.014068462 |
|---|
| InChI Key | IJOJIVNDFQSGAB-SQOUGZDYSA-N |
|---|
| InChI | InChI=1S/C6H11O9P/c7-3-2(1-14-16(11,12)13)15-6(10)5(9)4(3)8/h2-5,7-9H,1H2,(H2,11,12,13)/t2-,3-,4+,5-/m1/s1 |
|---|
| IUPAC Name | {[(2R,3S,4S,5R)-3,4,5-trihydroxy-6-oxooxan-2-yl]methoxy}phosphonic acid |
|---|
| Traditional IUPAC Name | 6-phosphogluconolactone |
|---|
| Chemical Formula | C6H11O9P |
|---|
| SMILES | [H]O[C@@]1([H])C(=O)O[C@]([H])(C([H])([H])OP(=O)(O[H])O[H])[C@@]([H])(O[H])[C@]1([H])O[H] |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose phosphate
- Gluconolactone
- Monosaccharide phosphate
- Delta valerolactone
- Delta_valerolactone
- Monoalkyl phosphate
- Alkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Oxane
- Secondary alcohol
- Carboxylic acid ester
- Lactone
- Organoheterocyclic compound
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Oxacycle
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | - endoplasmic reticulum
- cytoplasm
|
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | Not Available |
|---|
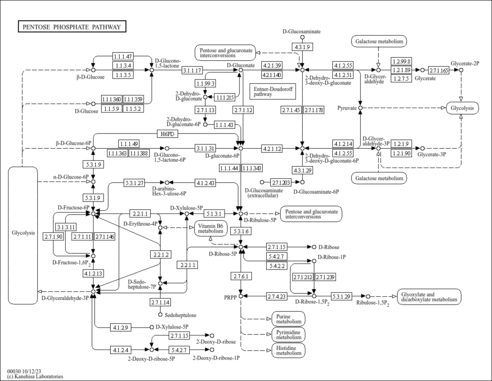
| KEGG Pathways | |
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9410000000-eed74c30dd7f6e1f6308 | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0gb9-4962700000-904ef84e4218dd793e1f | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-0490000000-b7b9499f5fb3885d4a30 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06r7-5940000000-effb433b86b6c959da1d | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ukc-7900000000-4009a042c78eba5dae17 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a6r-7290000000-09bacc885b7c74c16ece | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-5f41258b7bf2096e04fd | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-82b3c16e1939174dae85 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-5090000000-381acc8a668fbac0de38 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056s-9030000000-d9d231d9c36c323e6446 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-f53a8156649b945c2aee | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0900000000-8f89af904744e22d72b5 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-3900000000-8d2bf1400d030127126a | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9000000000-95f15475c3f10c3ac1bd | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - UniProt Consortium (2011). "Ongoing and future developments at the Universal Protein Resource." Nucleic Acids Res 39:D214-D219.21051339
- Herrgard, M. J., Swainston, N., Dobson, P., Dunn, W. B., Arga, K. Y., Arvas, M., Bluthgen, N., Borger, S., Costenoble, R., Heinemann, M., Hucka, M., Le Novere, N., Li, P., Liebermeister, W., Mo, M. L., Oliveira, A. P., Petranovic, D., Pettifer, S., Simeonidis, E., Smallbone, K., Spasic, I., Weichart, D., Brent, R., Broomhead, D. S., Westerhoff, H. V., Kirdar, B., Penttila, M., Klipp, E., Palsson, B. O., Sauer, U., Oliver, S. G., Mendes, P., Nielsen, J., Kell, D. B. (2008). "A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology." Nat Biotechnol 26:1155-1160.18846089
- Moir, R. D., Stokes, G. B. (1988). "A spectrophotometric assay for 6-phosphogluconolactonase involving the use of immobilized enzymes to prepare the labile 6-phosphoglucono-delta-lactone substrate." Biochem J 256:69-73.3223913
- KAWADA, M., KAGAWA, Y., TAKIGUCHI, H., SHIMAZONO, N. (1962). "Purification of 6-phosphogluconolactonase from rat liver and yeast; its separation from gluconolactonase." Biochim Biophys Acta 57:404-407.14454532
- Nogae, I., Johnston, M. (1990). "Isolation and characterization of the ZWF1 gene of Saccharomyces cerevisiae, encoding glucose-6-phosphate dehydrogenase." Gene 96:161-169.2269430
- Boer, V. M., Crutchfield, C. A., Bradley, P. H., Botstein, D., Rabinowitz, J. D. (2010). "Growth-limiting intracellular metabolites in yeast growing under diverse nutrient limitations." Mol Biol Cell 21:198-211.19889834
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | |
|---|