| Identification |
|---|
| YMDB ID | YMDB00096 |
|---|
| Name | (2R)-Homocitric acid |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Baker's yeast |
|---|
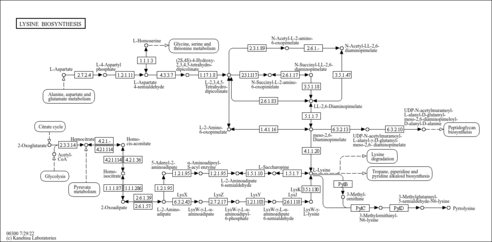
| Description | Homocitric acid, also known as homocitrate, belongs to the class of organic compounds known as tricarboxylic acids and derivatives. These are carboxylic acids containing exactly three carboxyl groups. Homocitric acid is an extremely weak basic (essentially neutral) compound (based on its pKa). Homocitric acid exists in all living species, ranging from bacteria to humans. Within yeast, homocitric acid participates in a number of enzymatic reactions. In particular, homocitric acid can be biosynthesized from oxoglutaric acid and acetyl-CoA through its interaction with the enzyme homocitrate synthase, mitochondrial. In addition, homocitric acid can be biosynthesized from oxoglutaric acid and acetyl-CoA; which is mediated by the enzyme homocitrate synthase, cytosolic isozyme. In yeast, homocitric acid is involved in the metabolic pathway called the lysine metabolism pathway. |
|---|
| Structure | |
|---|
| Synonyms | - (R)-2-Hydroxy-1,2,4-butanetricarboxylate
- (R)-2-Hydroxy-1,2,4-butanetricarboxylic acid
- (R)-2-Hydroxybutane-1,2,4-tricarboxylate
- (R)-2-hydroxybutane-1,2,4-tricarboxylic acid
- (R)-homocitric acid
- 2-Hydroxybutane-1,2,4-tricarboxylate
- 3-Hydroxy-3-carboxyadipate
- 3-Hydroxy-3-carboxyadipic acid
- Homocitrate
- Homocitric acid
- (R)-Homocitrate
- (2R)-2-Hydroxy-1,2,4-butanetricarboxylate
- (2R)-2-Hydroxy-1,2,4-butanetricarboxylic acid
- (±)-homocitrate
- (±)-homocitric acid
- 2-Hydroxy-1,2,4-butanetricarboxylate
- 2-Hydroxy-1,2,4-butanetricarboxylic acid
|
|---|
| CAS number | 3562-74-1 |
|---|
| Weight | Average: 206.1501
Monoisotopic: 206.042652674 |
|---|
| InChI Key | XKJVEVRQMLKSMO-SSDOTTSWSA-N |
|---|
| InChI | InChI=1S/C7H10O7/c8-4(9)1-2-7(14,6(12)13)3-5(10)11/h14H,1-3H2,(H,8,9)(H,10,11)(H,12,13)/t7-/m1/s1 |
|---|
| IUPAC Name | (2R)-2-hydroxybutane-1,2,4-tricarboxylic acid |
|---|
| Traditional IUPAC Name | (R)-homocitric acid |
|---|
| Chemical Formula | C7H10O7 |
|---|
| SMILES | OC(=O)CC[C@@](O)(CC(O)=O)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tricarboxylic acids and derivatives. These are carboxylic acids containing exactly three carboxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Tricarboxylic acids and derivatives |
|---|
| Direct Parent | Tricarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tricarboxylic acid or derivatives
- Hydroxy acid
- Alpha-hydroxy acid
- Tertiary alcohol
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | |
|---|
| KEGG Pathways | |
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fsr-2900000000-1b6221744212821d13a4 | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-004i-6039700000-bbcdc859874cb31d16db | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06ya-0910000000-10ce2a6c7dde8f6414f9 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03kc-6900000000-cc774f0962c580270a86 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-3900000000-1dbf32a3d216327d832a | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-08fr-1910000000-24186d115834b7a98219 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-07vi-3900000000-78ce979f1c8cb053a6d2 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aor-9600000000-dc1759e8907b4db138bd | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-07wl-1910000000-93d90a9e9b47624dde71 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014r-2900000000-10b9bd64cc7e5be41000 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ku-9800000000-3926d2bdbaae25830936 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052u-0930000000-b304c640e74bcbd68b82 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9200000000-f4181d391bb21114c3e1 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9200000000-28608fc4dea9afb51354 | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - UniProt Consortium (2011). "Ongoing and future developments at the Universal Protein Resource." Nucleic Acids Res 39:D214-D219.21051339
- Scheer, M., Grote, A., Chang, A., Schomburg, I., Munaretto, C., Rother, M., Sohngen, C., Stelzer, M., Thiele, J., Schomburg, D. (2011). "BRENDA, the enzyme information system in 2011." Nucleic Acids Res 39:D670-D676.21062828
- Herrgard, M. J., Swainston, N., Dobson, P., Dunn, W. B., Arga, K. Y., Arvas, M., Bluthgen, N., Borger, S., Costenoble, R., Heinemann, M., Hucka, M., Le Novere, N., Li, P., Liebermeister, W., Mo, M. L., Oliveira, A. P., Petranovic, D., Pettifer, S., Simeonidis, E., Smallbone, K., Spasic, I., Weichart, D., Brent, R., Broomhead, D. S., Westerhoff, H. V., Kirdar, B., Penttila, M., Klipp, E., Palsson, B. O., Sauer, U., Oliver, S. G., Mendes, P., Nielsen, J., Kell, D. B. (2008). "A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology." Nat Biotechnol 26:1155-1160.18846089
- Maragoudakis, M. E., Strassman, M. (1966). "Homocitric acid accumulation by a lysine-requiring yeast mutant." J Biol Chem 241:695-699.5908136
- Strassman, M., Ceci, L. N. (1965). "Enzymatic formation of alpha-ketoadipic acid from homoisocitric acid." J Biol Chem 240:4357-4361.4284830
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | |
|---|