| Identification |
|---|
| YMDB ID | YMDB13972 |
|---|
| Name | PE-NMe(15:1(9Z)/20:1(11Z)) |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Brewer's yeast |
|---|
| Description | PE-NMe(15:1(9Z)/20:1(11Z)) is a monomethylphosphatidylethanolamine. It is a glycerophospholipid, and is formed by sequential methylation of phosphatidylethanolamine as part of a mechanism for biosynthesis of phosphatidylcholine. Monomethylphosphatidylethanolamines are usually found at trace levels in animal or plant tissues. They can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. PE-NMe(15:1(9Z)/20:1(11Z)), in particular, consists of one 9Z-pentadecenoyl chain to the C-1 atom, and one 11Z-eicosenoyl to the C-2 atom. Fatty acids containing 16, 18 and 20 carbons are the most common. Phospholipids, are ubiquitous in nature and are key components of the lipid bilayer of cells, as well as being involved in metabolism and signaling. |
|---|
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| CAS number | Not Available |
|---|
| Weight | Average: 744.048
Monoisotopic: 743.546505474 |
|---|
| InChI Key | UKUFJAKAIIGUPN-WITMPDRSSA-N |
|---|
| InChI | InChI=1S/C41H78NO8P/c1-4-6-8-10-12-14-16-18-19-20-21-22-24-26-28-30-32-34-41(44)50-39(38-49-51(45,46)48-36-35-42-3)37-47-40(43)33-31-29-27-25-23-17-15-13-11-9-7-5-2/h13,15,18-19,39,42H,4-12,14,16-17,20-38H2,1-3H3,(H,45,46)/b15-13-,19-18- |
|---|
| IUPAC Name | {2-[(11Z)-icos-11-enoyloxy]-3-[(9Z)-pentadec-9-enoyloxy]propoxy}[2-(methylamino)ethoxy]phosphinic acid |
|---|
| Traditional IUPAC Name | 2-[(11Z)-icos-11-enoyloxy]-3-[(9Z)-pentadec-9-enoyloxy]propoxy(2-(methylamino)ethoxy)phosphinic acid |
|---|
| Chemical Formula | C41H78NO8P |
|---|
| SMILES | [H]C(COC(=O)CCCCCCC\C=C/CCCCC)(COP(O)(=O)OCCNC)OC(=O)CCCCCCCCC\C=C/CCCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | - Endoplasmic reticulum
- Mitochondria
|
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | | Phosphatidylcholine biosynthesis PC(15:1(9Z)/20:1(11Z)) | PW002919 | 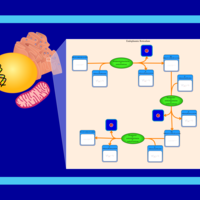 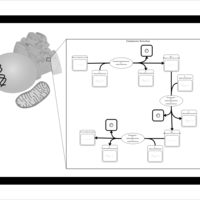 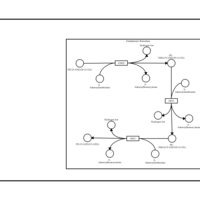 |
|
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9040110100-bb0b3c79f776391e47ab | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9140001000-0dd235bf6861f1232c94 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9170011000-424bf586146d7d92536e | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-007c-1192201200-c40f81f25fac301ce3cf | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fg9-6491100000-96432f6a804c518eaace | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-9110000000-4ca67b237db21428ccaf | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Rattray JB, Schibeci A, Kidby DK. (1975). "Lipids of yeasts." Bacteriol Rev. 1975 Sep;39(3):197-231.240350
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | FOODB ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|
