D-glyceraldehyde (YMDB00575)
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YMDB ID | YMDB00575 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name | D-glyceraldehyde | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Saccharomyces cerevisiae | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Strain | Baker's yeast | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | D-glyceraldehyde, also known as D-aldotriose or D-glycerose, belongs to the class of organic compounds known as monosaccharides. Monosaccharides are compounds containing one carbohydrate unit not glycosidically linked to another such unit, and no set of two or more glycosidically linked carbohydrate units. Monosaccharides have the general formula CnH2nOn. D-glyceraldehyde is an extremely weak basic (essentially neutral) compound (based on its pKa). D-glyceraldehyde may be a unique S. cerevisiae (yeast) metabolite. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 453-17-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | Average: 90.0779 Monoisotopic: 90.031694058 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | MNQZXJOMYWMBOU-VKHMYHEASA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI | InChI=1S/C3H6O3/c4-1-3(6)2-5/h1,3,5-6H,2H2/t3-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | (2R)-2,3-dihydroxypropanal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name | triose | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C3H6O3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [H]C(=O)[C@H](O)CO | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as monosaccharides. Monosaccharides are compounds containing one carbohydrate unit not glycosidically linked to another such unit, and no set of two or more glycosidically linked carbohydrate units. Monosaccharides have the general formula CnH2nOn. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic oxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Monosaccharides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 145 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Organoleptic Properties | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
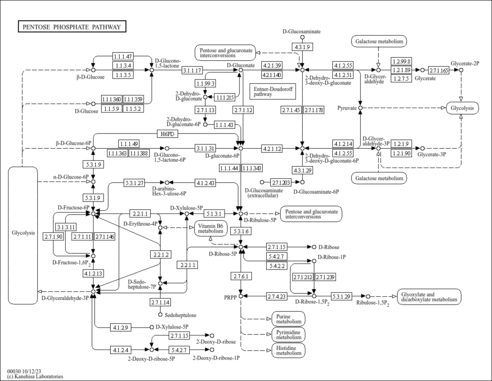
| KEGG Pathways |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Reactions | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Reactions |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intracellular Concentrations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Extracellular Concentrations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the aldol condensation of dihydroxyacetone phosphate (DHAP or glycerone-phosphate) with glyceraldehyde 3- phosphate (G3P) to form fructose 1,6-bisphosphate (FBP) in gluconeogenesis and the reverse reaction in glycolysis
- Gene Name:
- FBA1
- Uniprot ID:
- P14540
- Molecular weight:
- 39620.5
Reactions
| D-fructose 1,6-bisphosphate → glycerone phosphate + D-glyceraldehyde 3-phosphate. |
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the irreversible reduction of the cytotoxic compound methylglyoxal (MG) to (R)-lactaldehyde as an alternative to detoxification of MG by glyoxalase I GLO1. MG is synthesized via a bypath of glycolysis from dihydroxyacetone phosphate and is believed to play a role in cell cycle regulation and stress adaptation
- Gene Name:
- GRE2
- Uniprot ID:
- Q12068
- Molecular weight:
- 38169.19922
Reactions
| Lactaldehyde + NADP(+) → methylglyoxal + NADPH. |
| 3-methylbutanol + NAD(P)+ → 3-methylbutanal + NAD(P)H + H+ |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- Reduces the cytotoxic compound methylglyoxal (MG) to (R)-lactaldehyde similar to GRE2. MG is synthesized via a bypath of glycolysis from dihydroxyacetone phosphate and is believed to play a role in cell cycle regulation and stress adaptation. In pentose-fermenting yeasts, aldose reductase catalyzes the reduction of xylose into xylitol. The purified enzyme catalyzes this reaction, but the inability of S.cerevisiae to grow on xylose as sole carbon source indicates that the physiological function is more likely methylglyoxal reduction
- Gene Name:
- GRE3
- Uniprot ID:
- P38715
- Molecular weight:
- 37118.5
Reactions
| Alditol + NAD(P)(+) → aldose + NAD(P)H. |
| (R)-lactaldehyde + NADP(+) → methylglyoxal + NADPH. |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- Not Available
- Gene Name:
- YPR1
- Uniprot ID:
- Q12458
- Molecular weight:
- 34754.69922