| Identification |
|---|
| YMDB ID | YMDB00464 |
|---|
| Name | (S)-3-hydroxydecanoyl-CoA |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Baker's yeast |
|---|
| Description | (S)-3-hydroxydecanoyl-CoA, also known as 3-OH 10:0-CoA or 3-hydroxydecanoyl-coenzyme A, belongs to the class of organic compounds known as (s)-3-hydroxyacyl coas. These are organic compounds containing a (S)-3-hydroxyl acylated coenzyme A derivative. Based on a literature review a small amount of articles have been published on (S)-3-hydroxydecanoyl-CoA. |
|---|
| Structure | |
|---|
| Synonyms | - (S)-3-Hydroxydecanoyl-CoA
- (S)-3-Hydroxydecanoyl-Coenzyme A
- (S)-Hydroxydecanoyl-CoA
- 3-Hydroxydecanoyl-CoA
- 3-Hydroxydecanoyl-Coenzyme A
- 3-OH 10:0-CoA
- 3-OH C10:0-CoA
- 3S-hydroxy-decanoyl-CoA
- 3S-hydroxy-decanoyl-Coenzyme A
- b-Hydroxydecanoyl coenzyme A
- beta-Hydroxydecanoyl coenzyme A
- beta-hydroxydecanoyl-CoA
- coenzyme A S-3-hydroxydecanoate
- DL-b-Hydroxydecanoyl coenzyme A
- DL-beta-Hydroxydecanoyl coenzyme A
- S-(3-hydroxydecanoate
- S-(3-hydroxydecanoate) CoA
- S-(3-hydroxydecanoate) Coenzyme A
- S-(3-hydroxydecanoic acid
- S-(3-Hydroxydecanoyl)coenzyme A
- Β-hydroxydecanoyl coenzyme A
- b-Hydroxydecanoyl-CoA
- Β-hydroxydecanoyl-CoA
- coenzyme A S-3-Hydroxydecanoic acid
- 3-Hydroxydecanoyl-coenzyme A, (R)-isomer
|
|---|
| CAS number | 6245-70-1 |
|---|
| Weight | Average: 937.783
Monoisotopic: 937.245888185 |
|---|
| InChI Key | HIVSMYZAMUNFKZ-PNPVFPMQSA-N |
|---|
| InChI | InChI=1S/C31H54N7O18P3S/c1-4-5-6-7-8-9-19(39)14-22(41)60-13-12-33-21(40)10-11-34-29(44)26(43)31(2,3)16-53-59(50,51)56-58(48,49)52-15-20-25(55-57(45,46)47)24(42)30(54-20)38-18-37-23-27(32)35-17-36-28(23)38/h17-20,24-26,30,39,42-43H,4-16H2,1-3H3,(H,33,40)(H,34,44)(H,48,49)(H,50,51)(H2,32,35,36)(H2,45,46,47)/t19-,20+,24+,25+,26-,30+/m0/s1 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[(3R)-3-hydroxy-3-({2-[(2-{[(3S)-3-hydroxydecanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional IUPAC Name | (S)-3-hydroxydecanoyl-coa |
|---|
| Chemical Formula | C31H54N7O18P3S |
|---|
| SMILES | CCCCCCC[C@H](O)CC(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as (s)-3-hydroxyacyl coas. These are organic compounds containing a (S)-3-hydroxyl acylated coenzyme A derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | (S)-3-hydroxyacyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Monosaccharide
- Pyrimidine
- Alkyl phosphate
- Fatty amide
- Phosphoric acid ester
- Tetrahydrofuran
- Imidazole
- Azole
- Heteroaromatic compound
- Carbothioic s-ester
- Secondary alcohol
- Thiocarboxylic acid ester
- Carboxamide group
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Organosulfur compound
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Primary amine
- Organopnictogen compound
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | - lipid particle
- endoplasmic reticulum
- peroxisome
|
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | |
|---|
| KEGG Pathways | | Fatty acid elongation in mitochondria | ec00062 | 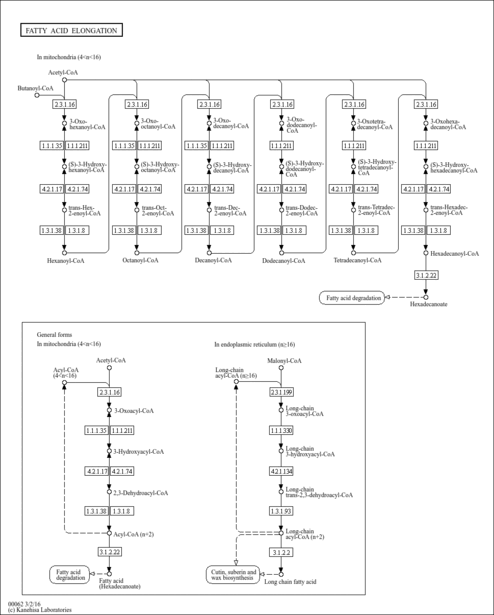 | | Fatty acid metabolism | ec00071 |  |
|
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions | |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1955100204-d6525c792d0660eed578 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000j-1962200000-a6554b2032895e40ea2f | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-2931000000-994000eb5c23a4a22111 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-017i-2901021304-e79be825caf02b5a8633 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-2900210001-793115062cfccb42901d | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-7900000000-3c7fb40503eb64a75147 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0000000009-6ce5bb10296fb5962d7f | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0170-3200101229-19c5c4b5d884bb6971bc | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00p3-3403501934-b1b23288f3869f2596e0 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0000000009-72e71f0413c0c2a433c3 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1600100197-d4e309ba205f695a9bcb | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0001900000-ddc361c28129c2b8128e | JSpectraViewer | | MS | Mass Spectrum (Electron Ionization) | Not Available | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Herrgard, M. J., Swainston, N., Dobson, P., Dunn, W. B., Arga, K. Y., Arvas, M., Bluthgen, N., Borger, S., Costenoble, R., Heinemann, M., Hucka, M., Le Novere, N., Li, P., Liebermeister, W., Mo, M. L., Oliveira, A. P., Petranovic, D., Pettifer, S., Simeonidis, E., Smallbone, K., Spasic, I., Weichart, D., Brent, R., Broomhead, D. S., Westerhoff, H. V., Kirdar, B., Penttila, M., Klipp, E., Palsson, B. O., Sauer, U., Oliver, S. G., Mendes, P., Nielsen, J., Kell, D. B. (2008). "A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology." Nat Biotechnol 26:1155-1160.18846089
|
|---|
| Synthesis Reference: | Qi, Q.; Steinbuchel, A.; Rehm, B. H. A. In vitro synthesis of poly(3-hydroxydecanoate): purification and enzymatic characterization of type II polyhydroxyalkanoate synthases PhaC1 and PhaC2 from Pseudomonas aeruginosa. Applied Microbiology and Biotech |
|---|
| External Links: | |
|---|


