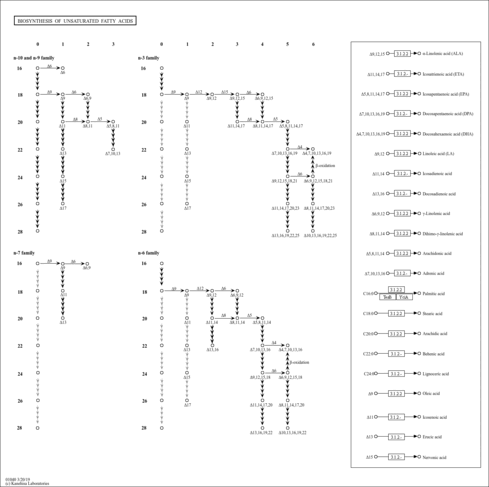
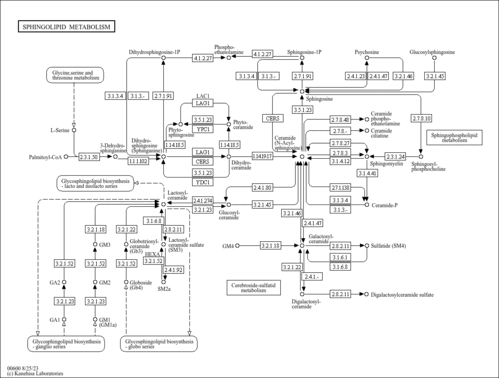
| General Reference | - Buede, R., Rinker-Schaffer, C., Pinto, W. J., Lester, R. L., Dickson, R. C. (1991). "Cloning and characterization of LCB1, a Saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids." J Bacteriol 173:4325-4332.2066332
- Bowman, S., Churcher, C., Badcock, K., Brown, D., Chillingworth, T., Connor, R., Dedman, K., Devlin, K., Gentles, S., Hamlin, N., Hunt, S., Jagels, K., Lye, G., Moule, S., Odell, C., Pearson, D., Rajandream, M., Rice, P., Skelton, J., Walsh, S., Whitehead, S., Barrell, B. (1997). "The nucleotide sequence of Saccharomyces cerevisiae chromosome XIII." Nature 387:90-93.9169872
- Hu, Y., Rolfs, A., Bhullar, B., Murthy, T. V., Zhu, C., Berger, M. F., Camargo, A. A., Kelley, F., McCarron, S., Jepson, D., Richardson, A., Raphael, J., Moreira, D., Taycher, E., Zuo, D., Mohr, S., Kane, M. F., Williamson, J., Simpson, A., Bulyk, M. L., Harlow, E., Marsischky, G., Kolodner, R. D., LaBaer, J. (2007). "Approaching a complete repository of sequence-verified protein-encoding clones for Saccharomyces cerevisiae." Genome Res 17:536-543.17322287
- Nagiec, M. M., Baltisberger, J. A., Wells, G. B., Lester, R. L., Dickson, R. C. (1994). "The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis." Proc Natl Acad Sci U S A 91:7899-7902.8058731
- Gable, K., Slife, H., Bacikova, D., Monaghan, E., Dunn, T. M. (2000). "Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity." J Biol Chem 275:7597-7603.10713067
- Gable, K., Han, G., Monaghan, E., Bacikova, D., Natarajan, M., Williams, R., Dunn, T. M. (2002). "Mutations in the yeast LCB1 and LCB2 genes, including those corresponding to the hereditary sensory neuropathy type I mutations, dominantly inactivate serine palmitoyltransferase." J Biol Chem 277:10194-10200.11781309
- Huh, W. K., Falvo, J. V., Gerke, L. C., Carroll, A. S., Howson, R. W., Weissman, J. S., O'Shea, E. K. (2003). "Global analysis of protein localization in budding yeast." Nature 425:686-691.14562095
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., Weissman, J. S. (2003). "Global analysis of protein expression in yeast." Nature 425:737-741.14562106
- Han, G., Gable, K., Yan, L., Natarajan, M., Krishnamurthy, J., Gupta, S. D., Borovitskaya, A., Harmon, J. M., Dunn, T. M. (2004). "The topology of the Lcb1p subunit of yeast serine palmitoyltransferase." J Biol Chem 279:53707-53716.15485854
- Li, X., Gerber, S. A., Rudner, A. D., Beausoleil, S. A., Haas, W., Villen, J., Elias, J. E., Gygi, S. P. (2007). "Large-scale phosphorylation analysis of alpha-factor-arrested Saccharomyces cerevisiae." J Proteome Res 6:1190-1197.17330950
- Breslow, D. K., Collins, S. R., Bodenmiller, B., Aebersold, R., Simons, K., Shevchenko, A., Ejsing, C. S., Weissman, J. S. (2010). "Orm family proteins mediate sphingolipid homeostasis." Nature 463:1048-1053.20182505
|
|---|