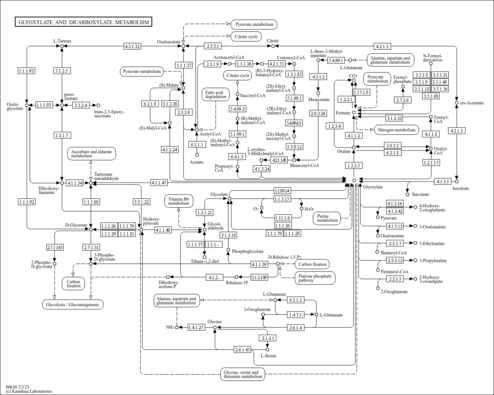
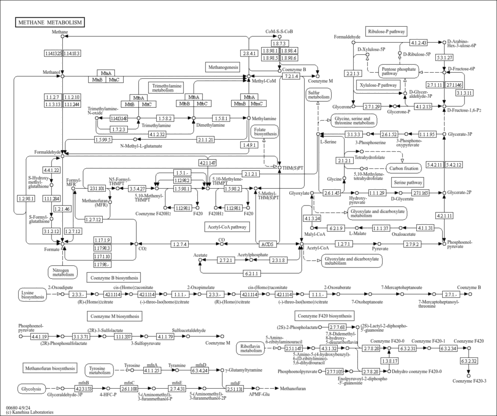
| General Reference | - Minard, K. I., McAlister-Henn, L. (1991). "Isolation, nucleotide sequence analysis, and disruption of the MDH2 gene from Saccharomyces cerevisiae: evidence for three isozymes of yeast malate dehydrogenase." Mol Cell Biol 11:370-380.1986231
- Casamayor, A., Khalid, H., Balcells, L., Aldea, M., Casas, C., Herrero, E., Arino, J. (1996). "Sequence analysis of a 13.4 kbp fragment from the left arm of chromosome XV reveals a malate dehydrogenase gene, a putative Ser/Thr protein kinase, the ribosomal L25 gene and four new open reading frames." Yeast 12:1013-1020.8896265
- Dujon, B., Albermann, K., Aldea, M., Alexandraki, D., Ansorge, W., Arino, J., Benes, V., Bohn, C., Bolotin-Fukuhara, M., Bordonne, R., Boyer, J., Camasses, A., Casamayor, A., Casas, C., Cheret, G., Cziepluch, C., Daignan-Fornier, B., Dang, D. V., de Haan, M., Delius, H., Durand, P., Fairhead, C., Feldmann, H., Gaillon, L., Kleine, K., et, a. l. .. (1997). "The nucleotide sequence of Saccharomyces cerevisiae chromosome XV." Nature 387:98-102.9169874
- Kopetzki, E., Entian, K. D., Lottspeich, F., Mecke, D. (1987). "Purification procedure and N-terminal amino acid sequence of yeast malate dehydrogenase isoenzymes." Biochim Biophys Acta 912:398-403.3552052
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., Weissman, J. S. (2003). "Global analysis of protein expression in yeast." Nature 425:737-741.14562106
- Albuquerque, C. P., Smolka, M. B., Payne, S. H., Bafna, V., Eng, J., Zhou, H. (2008). "A multidimensional chromatography technology for in-depth phosphoproteome analysis." Mol Cell Proteomics 7:1389-1396.18407956
|
|---|