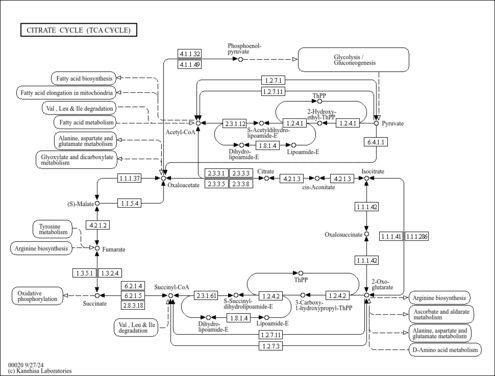
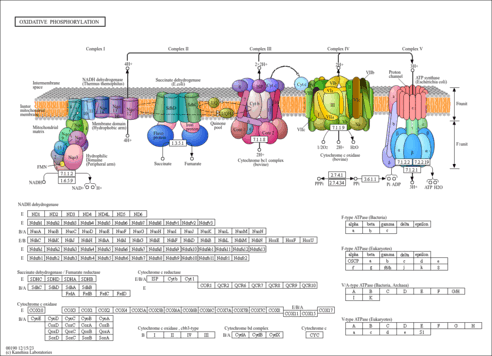
| General Reference | - Lombardo, A., Carine, K., Scheffler, I. E. (1990). "Cloning and characterization of the iron-sulfur subunit gene of succinate dehydrogenase from Saccharomyces cerevisiae." J Biol Chem 265:10419-10423.2191948
- Johnston, M., Hillier, L., Riles, L., Albermann, K., Andre, B., Ansorge, W., Benes, V., Bruckner, M., Delius, H., Dubois, E., Dusterhoft, A., Entian, K. D., Floeth, M., Goffeau, A., Hebling, U., Heumann, K., Heuss-Neitzel, D., Hilbert, H., Hilger, F., Kleine, K., Kotter, P., Louis, E. J., Messenguy, F., Mewes, H. W., Hoheisel, J. D., et, a. l. .. (1997). "The nucleotide sequence of Saccharomyces cerevisiae chromosome XII." Nature 387:87-90.9169871
- Hu, Y., Rolfs, A., Bhullar, B., Murthy, T. V., Zhu, C., Berger, M. F., Camargo, A. A., Kelley, F., McCarron, S., Jepson, D., Richardson, A., Raphael, J., Moreira, D., Taycher, E., Zuo, D., Mohr, S., Kane, M. F., Williamson, J., Simpson, A., Bulyk, M. L., Harlow, E., Marsischky, G., Kolodner, R. D., LaBaer, J. (2007). "Approaching a complete repository of sequence-verified protein-encoding clones for Saccharomyces cerevisiae." Genome Res 17:536-543.17322287
- Gould, S. J., Subramani, S., Scheffler, I. E. (1989). "Use of the DNA polymerase chain reaction for homology probing: isolation of partial cDNA or genomic clones encoding the iron-sulfur protein of succinate dehydrogenase from several species." Proc Natl Acad Sci U S A 86:1934-1938.2494655
- Schmidt, D. M., Saghbini, M., Scheffler, I. E. (1992). "The C-terminus of the succinate dehydrogenase IP peptide of Saccharomyces cerevisiae is significant for assembly of complex II." Biochemistry 31:8442-8448.1390628
- Lemire, B. D., Oyedotun, K. S. (2002). "The Saccharomyces cerevisiae mitochondrial succinate:ubiquinone oxidoreductase." Biochim Biophys Acta 1553:102-116.11803020
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., Weissman, J. S. (2003). "Global analysis of protein expression in yeast." Nature 425:737-741.14562106
- Oyedotun, K. S., Lemire, B. D. (2004). "The quaternary structure of the Saccharomyces cerevisiae succinate dehydrogenase. Homology modeling, cofactor docking, and molecular dynamics simulation studies." J Biol Chem 279:9424-9431.14672929
|
|---|