| Identification |
|---|
| YMDB ID | YMDB14374 |
|---|
| Name | PGP(16:0/16:1(9Z)) |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Brewer's yeast |
|---|
| Description | PGP(16:0/16:1(9Z)) belongs to the class of glycerophosphoglycerophosphates, also called phosphatidylglycerophosphates (PGPs). These lipids contain a common glycerophosphate skeleton linked to at least one fatty acyl chain and a glycero-3-phosphate moiety. As is the case with diacylglycerols, phosphatidylglycerophosphates can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PGP(16:0/16:1(9Z)), in particular, consists of one hexadecanoyl chain to the C-1 atom, and one 9Z-hexadecenoyl to the C-2 atom. In E. coli, PGPs can be found in the cytoplasmic membrane. The are synthesized by the addition of glycerol 3-phosphate to a CDP-diacylglycerol. In turn, PGPs are dephosphorylated to Phosphatidylglycerols (PGs) by the enzyme Phosphatidylglycerophosphatase. |
|---|
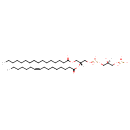
| Structure | |
|---|
| Synonyms | - 1-Palmitoyl-2-palmitoleoyl-sn-glycero-3-phospho-(1'-sn-glycerol-3'-phosphate)
- 3-sn-Phosphatidyl-1'-sn-glycerol 3'-phosphoric acid
- PGP(16:0/16:1)
- PGP(16:0/16:1n7)
- PGP(16:0/16:1W7)
- PGP(32:1)
- 1-Hexadecanoyl-2-(9Z-hexadecenoyl)-sn-glycero-3-phospho-(1'-sn-glycerol-3'-phosphate)
- PGP(16:0/16:1(9Z))
|
|---|
| CAS number | Not Available |
|---|
| Weight | Average: 800.945
Monoisotopic: 800.460466439 |
|---|
| InChI Key | RCAKEXBGCGLMOX-DCQLZOMZSA-N |
|---|
| InChI | InChI=1S/C38H74O13P2/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-37(40)47-33-36(34-50-53(45,46)49-32-35(39)31-48-52(42,43)44)51-38(41)30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h14,16,35-36,39H,3-13,15,17-34H2,1-2H3,(H,45,46)(H2,42,43,44)/b16-14-/t35-,36+/m0/s1 |
|---|
| IUPAC Name | [(2S)-3-({[(2R)-2-[(9Z)-hexadec-9-enoyloxy]-3-(hexadecanoyloxy)propoxy](hydroxy)phosphoryl}oxy)-2-hydroxypropoxy]phosphonic acid |
|---|
| Traditional IUPAC Name | (2S)-3-{[(2R)-2-[(9Z)-hexadec-9-enoyloxy]-3-(hexadecanoyloxy)propoxy(hydroxy)phosphoryl]oxy}-2-hydroxypropoxyphosphonic acid |
|---|
| Chemical Formula | C38H74O13P2 |
|---|
| SMILES | [H][C@](O)(COP(O)(O)=O)COP(O)(=O)OC[C@@]([H])(COC(=O)CCCCCCCCCCCCCCC)OC(=O)CCCCCCC\C=C/CCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylglycerophosphates. These are glycerophosphoglycerophosphates in which two fatty acids are bonded to the 1-glycerol moiety through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoglycerophosphates |
|---|
| Direct Parent | Phosphatidylglycerophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycerophosphoglycerophosphate
- Sn-glycerol-3-phosphate
- Fatty acid ester
- Monoalkyl phosphate
- Dialkyl phosphate
- Dicarboxylic acid or derivatives
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | | Cardiolipin Biosynthesis CL(16:0/16:1(9Z)/16:0/16:1(11Z)) | PW012522 |    | | Cardiolipin Biosynthesis CL(16:0/16:1(9Z)/16:0/16:1(9Z)) | PW012523 |    | | Cardiolipin Biosynthesis CL(16:0/16:1(9Z)/16:1(11Z)/16:1(11Z)) | PW012524 |    | | Cardiolipin Biosynthesis CL(16:0/16:1(9Z)/16:1(11Z)/16:1(9Z)) | PW012525 |    | | Cardiolipin Biosynthesis CL(16:0/16:1(9Z)/16:1(11Z)/18:0) | PW012526 |    |
|
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f6t-0490081530-55a138498138ecca372c | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-053b-1591041200-e03a93cd0a01f3741cf8 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052k-4893221100-deb0aa426adf6174ca2d | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-054t-3090030200-375cad12f6e125227779 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9050000000-a9baa34d7c44ba88a179 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9010000000-3d1708a487ebf6e3701b | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0010000900-1cf4f222fa3eff080c40 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-3091041400-b602219be812461b192d | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fba-3092061100-e4abaaab020b6afc20eb | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2100062690-19d4b58b1fbdb56421e8 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0k92-5600097400-2867fb8cdab51007291e | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k92-7496620000-c4d7ddfc0dce89035ba5 | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Rattray JB, Schibeci A, Kidby DK. (1975). "Lipids of yeasts." Bacteriol Rev. 1975 Sep;39(3):197-231.240350
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB0013473 | | Pubchem Compound ID | 53481795 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | FOODB ID | FDB029473 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|