| Identification |
|---|
| YMDB ID | YMDB14168 |
|---|
| Name | PGP(10:0/20:1(13Z)) |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Brewer's yeast |
|---|
| Description | PGP(10:0/20:1(13Z)) belongs to the class of glycerophosphoglycerophosphates, also called phosphatidylglycerophosphates (PGPs). These lipids contain a common glycerophosphate skeleton linked to at least one fatty acyl chain and a glycero-3-phosphate moiety. As is the case with diacylglycerols, phosphatidylglycerophosphates can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PGP(10:0/20:1(13Z)), in particular, consists of one decanoyl chain to the C-1 atom, and one 13Z-eicosenoyl to the C-2 atom. In E. coli, PGPs can be found in the cytoplasmic membrane. The are synthesized by the addition of glycerol 3-phosphate to a CDP-diacylglycerol. In turn, PGPs are dephosphorylated to Phosphatidylglycerols (PGs) by the enzyme Phosphatidylglycerophosphatase. |
|---|
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| CAS number | Not Available |
|---|
| Weight | Average: 772.891
Monoisotopic: 772.42916631 |
|---|
| InChI Key | OSABDHYPOOYGBV-REUHNWMUSA-N |
|---|
| InChI | InChI=1S/C36H70O13P2/c1-3-5-7-9-11-12-13-14-15-16-17-18-19-20-22-24-26-28-36(39)49-34(31-45-35(38)27-25-23-21-10-8-6-4-2)32-48-51(43,44)47-30-33(37)29-46-50(40,41)42/h12-13,33-34,37H,3-11,14-32H2,1-2H3,(H,43,44)(H2,40,41,42)/b13-12-/t33-,34+/m0/s1 |
|---|
| IUPAC Name | [(2S)-3-({[(2R)-3-(decanoyloxy)-2-[(13Z)-icos-13-enoyloxy]propoxy](hydroxy)phosphoryl}oxy)-2-hydroxypropoxy]phosphonic acid |
|---|
| Traditional IUPAC Name | (2S)-3-{[(2R)-3-(decanoyloxy)-2-[(13Z)-icos-13-enoyloxy]propoxy(hydroxy)phosphoryl]oxy}-2-hydroxypropoxyphosphonic acid |
|---|
| Chemical Formula | C36H70O13P2 |
|---|
| SMILES | [H][C@](O)(COP(O)(O)=O)COP(O)(=O)OC[C@@]([H])(COC(=O)CCCCCCCCC)OC(=O)CCCCCCCCCCC\C=C/CCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphatidylglycerophosphates. These are glycerophosphoglycerophosphates in which two fatty acids are bonded to the 1-glycerol moiety through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphoglycerophosphates |
|---|
| Direct Parent | Phosphatidylglycerophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diacylglycerophosphoglycerophosphate
- Sn-glycerol-3-phosphate
- Dialkyl phosphate
- Monoalkyl phosphate
- Fatty acid ester
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | | Cardiolipin Biosynthesis CL(10:0/20:1(13Z)/10:0/20:1(11Z)) | PW005839 |   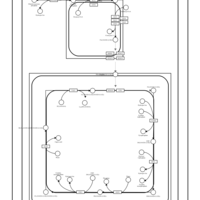 | | Cardiolipin Biosynthesis CL(10:0/20:1(13Z)/10:0/20:1(13Z)) | PW005840 |  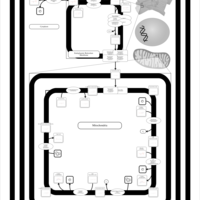 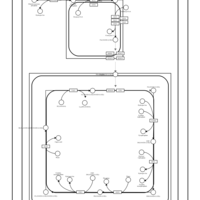 | | Cardiolipin Biosynthesis CL(10:0/20:1(13Z)/20:1(11Z)/20:1(11Z)) | PW005841 | 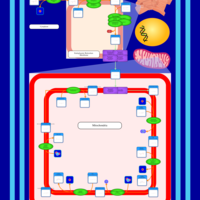 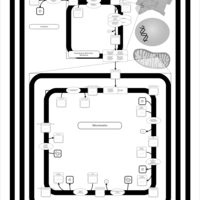 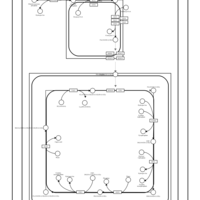 | | Cardiolipin Biosynthesis CL(10:0/20:1(13Z)/20:1(11Z)/20:1(13Z)) | PW005842 | 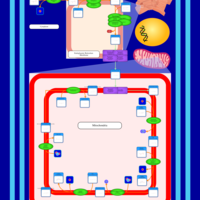  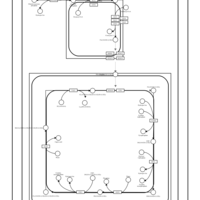 | | Cardiolipin Biosynthesis CL(10:0/20:1(13Z)/20:1(11Z)/22:0) | PW005843 |    |
|
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-1982424800-1bcce9e2c93395e21028 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-4891114200-35546429cc18c226661c | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-7694031000-d6ee0601fc20b9fc999b | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fmi-4922201300-fcd9440592847cc19bf4 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9410000000-89def1d46cf9f46f80ef | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-7354872edaf25b6c2dc5 | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Rattray JB, Schibeci A, Kidby DK. (1975). "Lipids of yeasts." Bacteriol Rev. 1975 Sep;39(3):197-231.240350
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | FOODB ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|
