| Identification |
|---|
| YMDB ID | YMDB12630 |
|---|
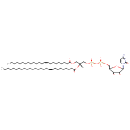
| Name | CDP-DG(23:1(9Z)/27:1(9Z)) |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Brewer's yeast |
|---|
| Description | CDP-DG(23:1(9Z)/27:1(9Z)) belongs to the family of CDP-diacylglycerols. It is a glycerophospholipid containing a diacylglycerol, with a cytidine diphosphate attached to the oxygen O1 or O2 of the glycerol part. As is the case with diacylglycerols, phosphatidylserines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. CDP-DG(23:1(9Z)/27:1(9Z)), in particular, consists of two 9Z-tricosanoyl chain at positions C-1 and C2. In E. coli glycerophospholipid metabolism, The biosynthesis of CDP-diacylglycerol (CDP-DG) involves condensation of phosphatidic acid (PA) and cytidine triphosphate, with elimination of pyrophosphate, catalysed by the enzyme CDP-diacylglycerol synthase. The resulting CDP-diacylglycerol can be utilized immediately for the synthesis of phosphatidylglycerol (PG), and thence cardiolipin (CL), and of phosphatidylinositol (PI). CDP-DG(23:1(9Z)/27:1(9Z)) is also a substrate of CDP-diacylglycerol pyrophosphatase. It is involved in CDP-diacylglycerol degradation pathway. |
|---|
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| CAS number | Not Available |
|---|
| Weight | Average: 1202.54
Monoisotopic: 1201.764693947 |
|---|
| InChI Key | LVJBPMCCWYNDQR-DOYMDUCCSA-N |
|---|
| InChI | InChI=1S/C62H113N3O15P2/c1-3-5-7-9-11-13-15-17-19-21-23-25-26-27-28-30-32-34-36-38-40-42-44-46-48-58(67)78-54(51-75-57(66)47-45-43-41-39-37-35-33-31-29-24-22-20-18-16-14-12-10-8-6-4-2)52-76-81(71,72)80-82(73,74)77-53-55-59(68)60(69)61(79-55)65-50-49-56(63)64-62(65)70/h31-34,49-50,54-55,59-61,68-69H,3-30,35-48,51-53H2,1-2H3,(H,71,72)(H,73,74)(H2,63,64,70)/b33-31-,34-32-/t54-,55-,59+,60?,61-/m1/s1 |
|---|
| IUPAC Name | {[(2R,3R,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}({[(2R)-2-[(9Z)-heptacos-9-enoyloxy]-3-[(9Z)-tricos-9-enoyloxy]propoxy](hydroxy)phosphoryl}oxy)phosphinic acid |
|---|
| Traditional IUPAC Name | [(2R,3R,5R)-5-(4-amino-2-oxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy([(2R)-2-[(9Z)-heptacos-9-enoyloxy]-3-[(9Z)-tricos-9-enoyloxy]propoxy(hydroxy)phosphoryl]oxy)phosphinic acid |
|---|
| Chemical Formula | C62H113N3O15P2 |
|---|
| SMILES | [H][C@@](COC(=O)CCCCCCC\C=C/CCCCCCCCCCCCC)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H](C(O)[C@H]1O)N1C=CC(N)=NC1=O)OC(=O)CCCCCCC\C=C/CCCCCCCCCCCCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as cdp-diacylglycerols. These are glycerolipids containing a diacylglycerol, with a cytidine diphosphate attached to the oxygen O1 or O2 of the glycerol part. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | CDP-glycerols |
|---|
| Direct Parent | CDP-diacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cdp-diacylglycerol
- Pyrimidine ribonucleoside diphosphate
- Diacyl-glycerol-3-pyrophosphate
- Pentose-5-phosphate
- Pentose phosphate
- N-glycosyl compound
- Glycosyl compound
- Pentose monosaccharide
- Organic pyrophosphate
- Monosaccharide phosphate
- Monoalkyl phosphate
- Pyrimidone
- Fatty acid ester
- Aminopyrimidine
- Fatty acyl
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Monosaccharide
- Hydropyrimidine
- Dicarboxylic acid or derivatives
- Heteroaromatic compound
- Tetrahydrofuran
- Secondary alcohol
- Carboxylic acid ester
- Amino acid or derivatives
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | | Cardiolipin Biosynthesis CL(10:0/10:0/23:1(9Z)/27:1(9Z)) | PW003706 |    | | Cardiolipin Biosynthesis CL(10:0/12:0/23:1(9Z)/27:1(9Z)) | PW003991 |    |
|
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0901000100-34a8cc3196d38ce65c4a | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1902000000-65de2a2e3e9722d6dca3 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-3902000000-6db9d53a2640d33942f6 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0pb9-0917200110-4e5c918c7541f4a093fc | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-1159-4609100001-df9c83e09b895e8a13ec | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bvi-5902000000-4f4aea2a62ba2e49385b | JSpectraViewer | | MS | Mass Spectrum (Electron Ionization) | Not Available | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Rattray JB, Schibeci A, Kidby DK. (1975). "Lipids of yeasts." Bacteriol Rev. 1975 Sep;39(3):197-231.240350
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | FOODB ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|