| Identification |
|---|
| YMDB ID | YMDB00530 |
|---|
| Name | lauroyl-CoA |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Baker's yeast |
|---|
| Description | Lauroyl-CoA, also known as dodecanoyl-CoA or C12:0-CoA, belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. Thus, lauroyl-CoA is considered to be a fatty ester lipid molecule. Lauroyl-CoA is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Lauroyl-CoA exists in all living species, ranging from bacteria to humans. |
|---|
| Structure | |
|---|
| Synonyms | - C12:0-CoA
- coenzyme A, S-dodecanoate
- Dodecanoyl-CoA
- Dodecanoyl-Coenzyme A
- lauroyl coenzyme a
- Lauroyl-CoA
- Lauroyl-coenzyme A
- coenzyme A, S-Dodecanoic acid
|
|---|
| CAS number | 6244-92-4 |
|---|
| Weight | Average: 949.837
Monoisotopic: 949.282273691 |
|---|
| InChI Key | YMCXGHLSVALICC-GMHMEAMDSA-N |
|---|
| InChI | InChI=1S/C33H58N7O17P3S/c1-4-5-6-7-8-9-10-11-12-13-24(42)61-17-16-35-23(41)14-15-36-31(45)28(44)33(2,3)19-54-60(51,52)57-59(49,50)53-18-22-27(56-58(46,47)48)26(43)32(55-22)40-21-39-25-29(34)37-20-38-30(25)40/h20-22,26-28,32,43-44H,4-19H2,1-3H3,(H,35,41)(H,36,45)(H,49,50)(H,51,52)(H2,34,37,38)(H2,46,47,48)/t22-,26-,27-,28+,32-/m1/s1 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-2-({[({[(3R)-3-[(2-{[2-(dodecanoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]oxy}methyl)-4-hydroxyoxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional IUPAC Name | lauroyl-coa |
|---|
| Chemical Formula | C33H58N7O17P3S |
|---|
| SMILES | CCCCCCCCCCCC(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-hydroxy acid
- Monocyclic benzene moiety
- Morpholine
- Oxazinane
- Piperidine
- N-alkylpyrrolidine
- Benzenoid
- Pyrrolidine
- Amino acid or derivatives
- Carboxylic acid ester
- Tertiary amine
- Tertiary aliphatic amine
- Oxacycle
- Organoheterocyclic compound
- Azacycle
- Carboxylic acid derivative
- Dialkyl ether
- Oxirane
- Ether
- Monocarboxylic acid or derivatives
- Primary alcohol
- Organopnictogen compound
- Organonitrogen compound
- Organooxygen compound
- Amine
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | - lipid particle
- endoplasmic reticulum
- peroxisome
- cytoplasm
|
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | |
|---|
| KEGG Pathways | | Fatty acid elongation in mitochondria | ec00062 | 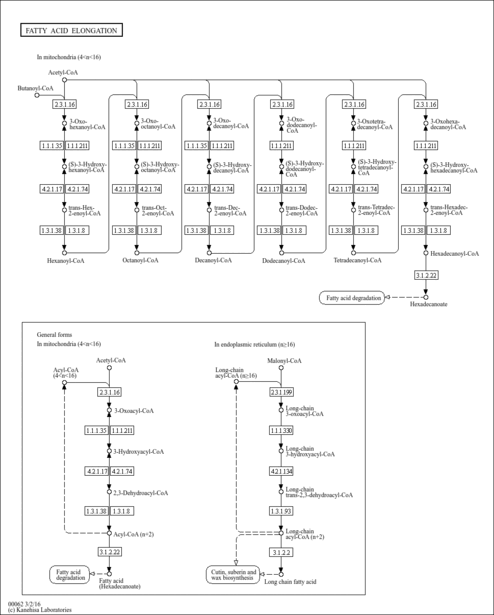 | | Fatty acid metabolism | ec00071 |  |
|
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions |
|
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1911101102-687968f22ae76ca547b2 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0931300000-f614ebfa62da3b2be8e6 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1910102000-031217cf76abed1b705f | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-003r-3922021304-259fa0e4c2115b5176b6 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-2901100001-efdce198394adecd9a34 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057i-6900100000-7e1a4d11b2e1da20a826 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0000000009-4ced9e4884f4bd265410 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0019-0901100447-ee848f28173670048ea9 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0001900000-d82983ad037a63e5050a | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000000009-c75a8c2363c43150b22a | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0032-4200302409-7fff9c03c40bd4272f8a | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-057r-5203701928-d7bda2a7e4eb819728f6 | JSpectraViewer | | MS | Mass Spectrum (Electron Ionization) | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Herrgard, M. J., Swainston, N., Dobson, P., Dunn, W. B., Arga, K. Y., Arvas, M., Bluthgen, N., Borger, S., Costenoble, R., Heinemann, M., Hucka, M., Le Novere, N., Li, P., Liebermeister, W., Mo, M. L., Oliveira, A. P., Petranovic, D., Pettifer, S., Simeonidis, E., Smallbone, K., Spasic, I., Weichart, D., Brent, R., Broomhead, D. S., Westerhoff, H. V., Kirdar, B., Penttila, M., Klipp, E., Palsson, B. O., Sauer, U., Oliver, S. G., Mendes, P., Nielsen, J., Kell, D. B. (2008). "A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology." Nat Biotechnol 26:1155-1160.18846089
- Jones, J. M., Nau, K., Geraghty, M. T., Erdmann, R., Gould, S. J. (1999). "Identification of peroxisomal acyl-CoA thioesterases in yeast and humans." J Biol Chem 274:9216-9223.10092594
|
|---|
| Synthesis Reference: | Eaton, Simon; Turnbull, Douglass M.; Bartlett, Kim. Redox control of b-oxidation in rat liver mitochondria. European Journal of Biochemistry (1994), 220(3), 671-81. |
|---|
| External Links: | |
|---|


