| Identification |
|---|
| YMDB ID | YMDB00272 |
|---|
| Name | D-4'-Phosphopantothenate |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Baker's yeast |
|---|
| Description | D-4'-Phosphopantothenate belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. D-4'-Phosphopantothenate is an extremely weak basic (essentially neutral) compound (based on its pKa). D-4'-Phosphopantothenate exists in all living species, ranging from bacteria to humans. Within yeast, D-4'-phosphopantothenate participates in a number of enzymatic reactions. In particular, D-4'-phosphopantothenate can be biosynthesized from pantothenic acid; which is mediated by the enzyme pantothenate kinase. In addition, D-4'-phosphopantothenate, cytidine triphosphate, and L-cysteine can be converted into cytidine monophosphate and 4'-phosphopantothenoylcysteine; which is mediated by the enzyme phosphopantothenate-cysteine ligase. In yeast, D-4'-phosphopantothenate is involved in the metabolic pathway called pantothenate and coa biosynthesis pathway. |
|---|
| Structure | |
|---|
| Synonyms | - (R)-4'-phosphopantothenate
- 4'-P-Pantothenate
- 4'-phosphopantothenate
- D-4'-Phosphopantothenate
- D-4'-Phosphopantothenic acid
- Phosphopantothenic acid
- Phosphopantothenic acid, calcium salt (2:1)
- Phosphopantothenic acid, calcium salt, (R)-isomer
|
|---|
| CAS number | Not Available |
|---|
| Weight | Average: 299.2149
Monoisotopic: 299.077003069 |
|---|
| InChI Key | XHFVGHPGDLDEQO-UHFFFAOYSA-N |
|---|
| InChI | InChI=1S/C9H18NO8P/c1-9(2,5-18-19(15,16)17)7(13)8(14)10-4-3-6(11)12/h7,13H,3-5H2,1-2H3,(H,10,14)(H,11,12)(H2,15,16,17) |
|---|
| IUPAC Name | 3-{2-hydroxy-3-methyl-3-[(phosphonooxy)methyl]butanamido}propanoic acid |
|---|
| Traditional IUPAC Name | 3-{2-hydroxy-3-methyl-3-[(phosphonooxy)methyl]butanamido}propanoic acid |
|---|
| Chemical Formula | C9H18NO8P |
|---|
| SMILES | [H]OC(=O)C([H])([H])C([H])([H])N([H])C(=O)C([H])(O[H])C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])OP(=O)(O[H])O[H] |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Beta amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta amino acid or derivatives
- Monoalkyl phosphate
- Fatty amide
- Monosaccharide
- N-acyl-amine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Fatty acyl
- Secondary alcohol
- Carboxamide group
- Secondary carboxylic acid amide
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic nitrogen compound
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | |
|---|
| KEGG Pathways | | Pantothenate and CoA biosynthesis | ec00770 | 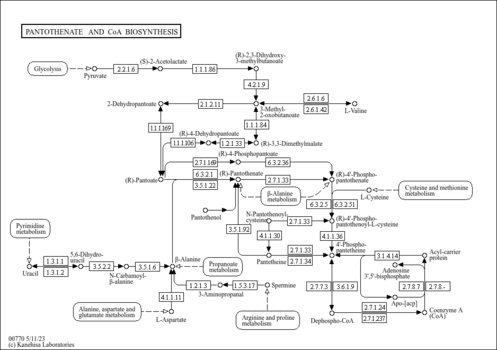 | | beta-Alanine metabolism | ec00410 |  |
|
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions | |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | |
|---|
| References |
|---|
| References: | - UniProt Consortium (2011). "Ongoing and future developments at the Universal Protein Resource." Nucleic Acids Res 39:D214-D219.21051339
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | |
|---|


