Lactaldehyde (YMDB00167)
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YMDB ID | YMDB00167 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name | Lactaldehyde | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Species | Saccharomyces cerevisiae | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Strain | Baker's yeast | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Lactaldehyde, also known as 2-hydroxypropanal, belongs to the class of organic compounds known as alpha-hydroxyaldehydes. These are organic compounds containing an aldehyde substituted with a hydroxyl group on the adjacent carbon. Lactaldehyde is an extremely weak basic (essentially neutral) compound (based on its pKa). Lactaldehyde exists in all living species, ranging from bacteria to humans. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 598-35-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight | Average: 74.0785 Monoisotopic: 74.036779436 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | BSABBBMNWQWLLU-VKHMYHEASA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI | InChI=1S/C3H6O2/c1-3(5)2-4/h2-3,5H,1H3/t3-/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | (2S)-2-hydroxypropanal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name | L-lactaldehyde | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C3H6O2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | [H]O[C@]([H])(C([H])=O)C([H])([H])[H] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as alpha-hydroxyaldehydes. These are organic compounds containing an aldehyde substituted with a hydroxyl group on the adjacent carbon. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic oxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Carbonyl compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Alpha-hydroxyaldehydes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Organoleptic Properties | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
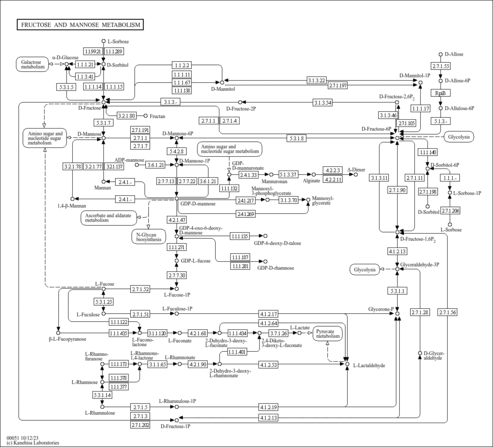
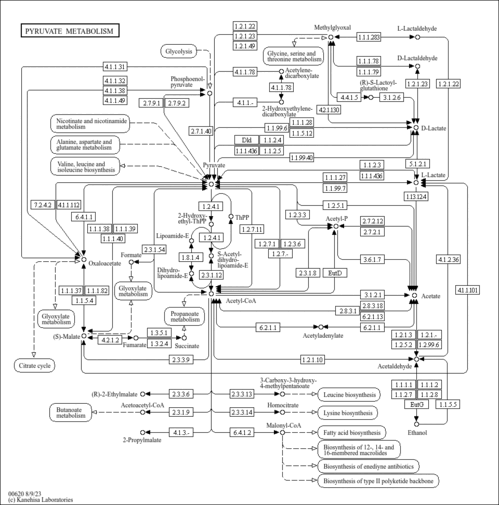
| KEGG Pathways |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Reactions |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Reactions |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Intracellular Concentrations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Extracellular Concentrations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Kranz, Cyrill. Synthesis of Lactic Aldehyde. Chemicke Listy pro Vedu a Prumysl (1912), 5 323-7. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Catalyzes the irreversible reduction of the cytotoxic compound methylglyoxal (MG) to (R)-lactaldehyde as an alternative to detoxification of MG by glyoxalase I GLO1. MG is synthesized via a bypath of glycolysis from dihydroxyacetone phosphate and is believed to play a role in cell cycle regulation and stress adaptation
- Gene Name:
- GRE2
- Uniprot ID:
- Q12068
- Molecular weight:
- 38169.19922
Reactions
| Lactaldehyde + NADP(+) → methylglyoxal + NADPH. |
| 3-methylbutanol + NAD(P)+ → 3-methylbutanal + NAD(P)H + H+ |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- Reduces the cytotoxic compound methylglyoxal (MG) to (R)-lactaldehyde similar to GRE2. MG is synthesized via a bypath of glycolysis from dihydroxyacetone phosphate and is believed to play a role in cell cycle regulation and stress adaptation. In pentose-fermenting yeasts, aldose reductase catalyzes the reduction of xylose into xylitol. The purified enzyme catalyzes this reaction, but the inability of S.cerevisiae to grow on xylose as sole carbon source indicates that the physiological function is more likely methylglyoxal reduction
- Gene Name:
- GRE3
- Uniprot ID:
- P38715
- Molecular weight:
- 37118.5
Reactions
| Alditol + NAD(P)(+) → aldose + NAD(P)H. |
| (R)-lactaldehyde + NADP(+) → methylglyoxal + NADPH. |