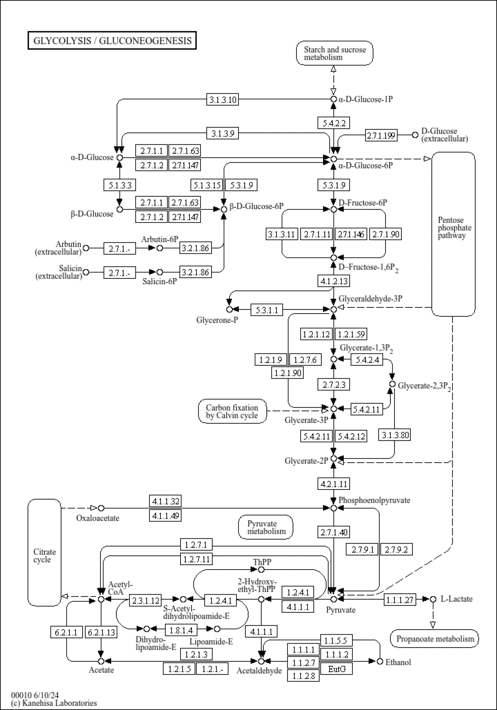
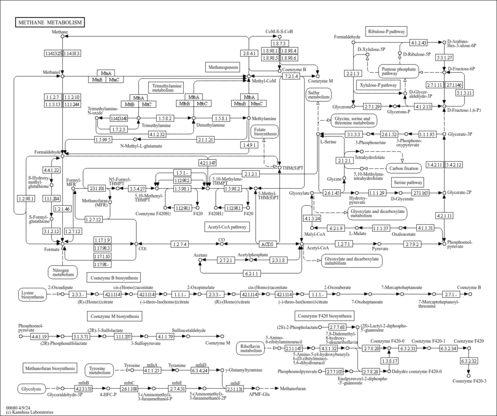
| General Reference | - Holland, M. J., Holland, J. P., Thill, G. P., Jackson, K. A. (1981). "The primary structures of two yeast enolase genes. Homology between the 5' noncoding flanking regions of yeast enolase and glyceraldehyde-3-phosphate dehydrogenase genes." J Biol Chem 256:1385-1395.6256394
- Johnston, M., Andrews, S., Brinkman, R., Cooper, J., Ding, H., Dover, J., Du, Z., Favello, A., Fulton, L., Gattung, S., et, a. l. .. (1994). "Complete nucleotide sequence of Saccharomyces cerevisiae chromosome VIII." Science 265:2077-2082.8091229
- Norbeck, J., Blomberg, A. (1996). "Protein expression during exponential growth in 0.7 M NaCl medium of Saccharomyces cerevisiae." FEMS Microbiol Lett 137:1-8.8935650
- Norbeck, J., Blomberg, A. (1995). "Gene linkage of two-dimensional polyacrylamide gel electrophoresis resolved proteins from isogene families in Saccharomyces cerevisiae by microsequencing of in-gel trypsin generated peptides." Electrophoresis 16:149-156.7737086
- Garrels, J. I., Futcher, B., Kobayashi, R., Latter, G. I., Schwender, B., Volpe, T., Warner, J. R., McLaughlin, C. S. (1994). "Protein identifications for a Saccharomyces cerevisiae protein database." Electrophoresis 15:1466-1486.7895733
- Ficarro, S. B., McCleland, M. L., Stukenberg, P. T., Burke, D. J., Ross, M. M., Shabanowitz, J., Hunt, D. F., White, F. M. (2002). "Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae." Nat Biotechnol 20:301-305.11875433
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., Weissman, J. S. (2003). "Global analysis of protein expression in yeast." Nature 425:737-741.14562106
- Gruhler, A., Olsen, J. V., Mohammed, S., Mortensen, P., Faergeman, N. J., Mann, M., Jensen, O. N. (2005). "Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway." Mol Cell Proteomics 4:310-327.15665377
- Chi, A., Huttenhower, C., Geer, L. Y., Coon, J. J., Syka, J. E., Bai, D. L., Shabanowitz, J., Burke, D. J., Troyanskaya, O. G., Hunt, D. F. (2007). "Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry." Proc Natl Acad Sci U S A 104:2193-2198.17287358
- Albuquerque, C. P., Smolka, M. B., Payne, S. H., Bafna, V., Eng, J., Zhou, H. (2008). "A multidimensional chromatography technology for in-depth phosphoproteome analysis." Mol Cell Proteomics 7:1389-1396.18407956
|
|---|