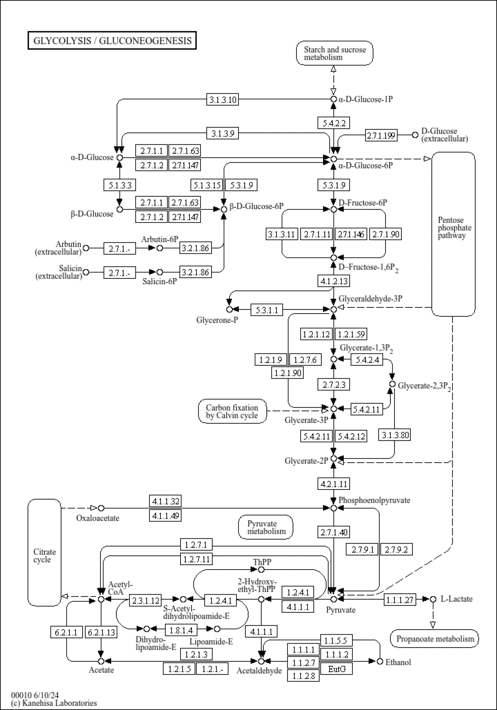
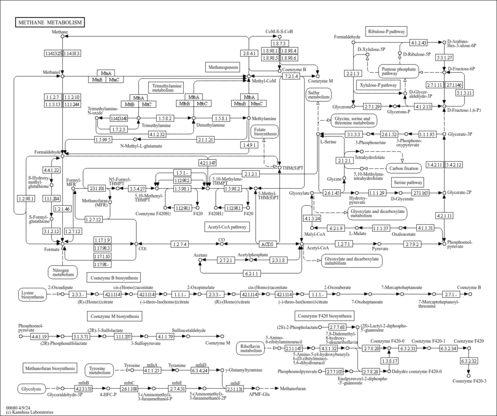
| General Reference | - Holland, M. J., Holland, J. P., Thill, G. P., Jackson, K. A. (1981). "The primary structures of two yeast enolase genes. Homology between the 5' noncoding flanking regions of yeast enolase and glyceraldehyde-3-phosphate dehydrogenase genes." J Biol Chem 256:1385-1395.6256394
- Mazzoni, C., Ruzzi, M., Rinaldi, T., Solinas, F., Montebove, F., Frontali, L. (1997). "Sequence analysis of a 10.5 kb DNA fragment from the yeast chromosome VII reveals the presence of three new open reading frames and of a tRNAThr gene." Yeast 13:369-372.9133741
- Tettelin, H., Agostoni Carbone, M. L., Albermann, K., Albers, M., Arroyo, J., Backes, U., Barreiros, T., Bertani, I., Bjourson, A. J., Bruckner, M., Bruschi, C. V., Carignani, G., Castagnoli, L., Cerdan, E., Clemente, M. L., Coblenz, A., Coglievina, M., Coissac, E., Defoor, E., Del Bino, S., Delius, H., Delneri, D., de Wergifosse, P., Dujon, B., Kleine, K., et, a. l. .. (1997). "The nucleotide sequence of Saccharomyces cerevisiae chromosome VII." Nature 387:81-84.9169869
- Chin, C. C., Brewer, J. M., Wold, F. (1981). "The amino acid sequence of yeast enolase." J Biol Chem 256:1377-1384.7005235
- Garrels, J. I., Futcher, B., Kobayashi, R., Latter, G. I., Schwender, B., Volpe, T., Warner, J. R., McLaughlin, C. S. (1994). "Protein identifications for a Saccharomyces cerevisiae protein database." Electrophoresis 15:1466-1486.7895733
- Norbeck, J., Blomberg, A. (1995). "Gene linkage of two-dimensional polyacrylamide gel electrophoresis resolved proteins from isogene families in Saccharomyces cerevisiae by microsequencing of in-gel trypsin generated peptides." Electrophoresis 16:149-156.7737086
- Poyner, R. R., Laughlin, L. T., Sowa, G. A., Reed, G. H. (1996). "Toward identification of acid/base catalysts in the active site of enolase: comparison of the properties of K345A, E168Q, and E211Q variants." Biochemistry 35:1692-1699.8634301
- Brewer, J. M., Holland, M. J., Lebioda, L. (2000). "The H159A mutant of yeast enolase 1 has significant activity." Biochem Biophys Res Commun 276:1199-1202.11027610
- Grandier-Vazeille, X., Bathany, K., Chaignepain, S., Camougrand, N., Manon, S., Schmitter, J. M. (2001). "Yeast mitochondrial dehydrogenases are associated in a supramolecular complex." Biochemistry 40:9758-9769.11502169
- Ficarro, S. B., McCleland, M. L., Stukenberg, P. T., Burke, D. J., Ross, M. M., Shabanowitz, J., Hunt, D. F., White, F. M. (2002). "Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae." Nat Biotechnol 20:301-305.11875433
- Brewer, J. M., Glover, C. V., Holland, M. J., Lebioda, L. (2003). "Enzymatic function of loop movement in enolase: preparation and some properties of H159N, H159A, H159F, and N207A enolases." J Protein Chem 22:353-361.13678299
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., Weissman, J. S. (2003). "Global analysis of protein expression in yeast." Nature 425:737-741.14562106
- Gruhler, A., Olsen, J. V., Mohammed, S., Mortensen, P., Faergeman, N. J., Mann, M., Jensen, O. N. (2005). "Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway." Mol Cell Proteomics 4:310-327.15665377
- Li, X., Gerber, S. A., Rudner, A. D., Beausoleil, S. A., Haas, W., Villen, J., Elias, J. E., Gygi, S. P. (2007). "Large-scale phosphorylation analysis of alpha-factor-arrested Saccharomyces cerevisiae." J Proteome Res 6:1190-1197.17330950
- Chi, A., Huttenhower, C., Geer, L. Y., Coon, J. J., Syka, J. E., Bai, D. L., Shabanowitz, J., Burke, D. J., Troyanskaya, O. G., Hunt, D. F. (2007). "Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry." Proc Natl Acad Sci U S A 104:2193-2198.17287358
- Smolka, M. B., Albuquerque, C. P., Chen, S. H., Zhou, H. (2007). "Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases." Proc Natl Acad Sci U S A 104:10364-10369.17563356
- Albuquerque, C. P., Smolka, M. B., Payne, S. H., Bafna, V., Eng, J., Zhou, H. (2008). "A multidimensional chromatography technology for in-depth phosphoproteome analysis." Mol Cell Proteomics 7:1389-1396.18407956
- Lebioda, L., Stec, B. (1988). "Crystal structure of enolase indicates that enolase and pyruvate kinase evolved from a common ancestor." Nature 333:683-686.3374614
- Lebioda, L., Stec, B., Brewer, J. M. (1989). "The structure of yeast enolase at 2.25-A resolution. An 8-fold beta + alpha-barrel with a novel beta beta alpha alpha (beta alpha)6 topology." J Biol Chem 264:3685-3693.2645275
- Stec, B., Lebioda, L. (1990). "Refined structure of yeast apo-enolase at 2.25 A resolution." J Mol Biol 211:235-248.2405163
- Larsen, T. M., Wedekind, J. E., Rayment, I., Reed, G. H. (1996). "A carboxylate oxygen of the substrate bridges the magnesium ions at the active site of enolase: structure of the yeast enzyme complexed with the equilibrium mixture of 2-phosphoglycerate and phosphoenolpyruvate at 1.8 A resolution." Biochemistry 35:4349-4358.8605183
- Zhang, E., Brewer, J. M., Minor, W., Carreira, L. A., Lebioda, L. (1997). "Mechanism of enolase: the crystal structure of asymmetric dimer enolase-2-phospho-D-glycerate/enolase-phosphoenolpyruvate at 2.0 A resolution." Biochemistry 36:12526-12534.9376357
- Poyner, R. R., Larsen, T. M., Wong, S. W., Reed, G. H. (2002). "Functional and structural changes due to a serine to alanine mutation in the active-site flap of enolase." Arch Biochem Biophys 401:155-163.12054465
- Sims, P. A., Larsen, T. M., Poyner, R. R., Cleland, W. W., Reed, G. H. (2003). "Reverse protonation is the key to general acid-base catalysis in enolase." Biochemistry 42:8298-8306.12846578
|
|---|