| Identification |
|---|
| YMDB ID | YMDB15973 |
|---|
| Name | Cadalene |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Baker's yeast |
|---|
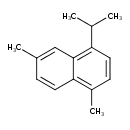
| Description | Cadalene (4-isopropyl-1,6-dimethylnaphthalene) is a polycyclic aromatic hydrocarbon with a chemical formula C15H18 and a cadinane skeleton. It is derived from generic sesquiterpenes, and ubiquitous in essential oils of many higher plants. Cadalene, together with retene, simonellite and ip-iHMN, is a biomarker of higher plants, which makes it useful for paleobotanic analysis of rock sediments. The ratio of retene to cadalene in sediments can reveal the ratio of the genus Pinaceae in the biosphere. (Wikipedia) |
|---|
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| CAS number | Not Available |
|---|
| Weight | Average: 198.3034
Monoisotopic: 198.140850576 |
|---|
| InChI Key | VMOJIHDTVZTGDO-UHFFFAOYSA-N |
|---|
| InChI | InChI=1S/C15H18/c1-10(2)13-8-6-12(4)14-7-5-11(3)9-15(13)14/h5-10H,1-4H3 |
|---|
| IUPAC Name | 1,6-dimethyl-4-(propan-2-yl)naphthalene |
|---|
| Traditional IUPAC Name | cadalene |
|---|
| Chemical Formula | C15H18 |
|---|
| SMILES | CC(C)C1=C2C=C(C)C=CC2=C(C)C=C1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Cadinane sesquiterpenoid
- Naphthalene
- Benzenoid
- Aromatic hydrocarbon
- Polycyclic hydrocarbon
- Unsaturated hydrocarbon
- Hydrocarbon
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | Not Available |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | Not Available |
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-053r-1900000000-e03a05ac04da9143239a | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-f2bd9379af6776ffe038 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-73c91ba9d394b3ebe92b | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05ai-2900000000-96f07179112485b07595 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-6d71e27487a53d2cadb9 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-06a8768f6d6deeea25b6 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053s-0900000000-a50f7e0957ce60590146 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052b-0900000000-11f56c8a69db4dfbc00a | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052b-0900000000-4cd13731e43e303e2027 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-6900000000-ad1d5c3528217c39c408 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-77af6775e009aeaad051 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0900000000-a8eed175446092f6c93d | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0900000000-4deafb7312bd6c9686ea | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Robinson AL, Ebeler SE, Heymann H, Boss PK, Solomon PS, Trengove RD. (2009). "Interactions between wine volatile compounds and grape and wine matrix components influence aroma compound headspace partitioning." J Agric Food Chem. 2009 Nov 11;57(21):10313-22.19845354
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | |
|---|