| Identification |
|---|
| YMDB ID | YMDB13533 |
|---|
| Name | PA(15:0/16:0) |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Brewer's yeast |
|---|
| Description | PA(15:0/16:0) is a phosphatidic acid. It is a glycerophospholipid in which a phosphate moiety occupies a glycerol substitution site. As is the case with diacylglycerols, phosphatidic acids can have many different combinations of fatty acids of varying lengths and saturation attached at the C-1 and C-2 positions. Fatty acids containing 16, 18 and 20 carbons are the most common. PA(15:0/16:0), in particular, consists of one pentadecanoyl chain to the C-1 atom, and one hexadecanoyl to the C-2 atom. The oleic acid moiety is derived from vegetable oils, especially olive and canola oil, while the oleic acid moiety is derived from vegetable oils, especially olive and canola oil. Phosphatidic acids are quite rare but are extremely important as intermediates in the biosynthesis of triacylglycerols and phospholipids. |
|---|
| Structure | |
|---|
| Synonyms | Not Available |
|---|
| CAS number | Not Available |
|---|
| Weight | Average: 634.876
Monoisotopic: 634.457356115 |
|---|
| InChI Key | UNPVCVLEAVYZII-JGCGQSQUSA-N |
|---|
| InChI | InChI=1S/C34H67O8P/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-34(36)42-32(31-41-43(37,38)39)30-40-33(35)28-26-24-22-20-18-16-14-12-10-8-6-4-2/h32H,3-31H2,1-2H3,(H2,37,38,39)/t32-/m1/s1 |
|---|
| IUPAC Name | [(2R)-2-(hexadecanoyloxy)-3-(pentadecanoyloxy)propoxy]phosphonic acid |
|---|
| Traditional IUPAC Name | (2R)-2-(hexadecanoyloxy)-3-(pentadecanoyloxy)propoxyphosphonic acid |
|---|
| Chemical Formula | C34H67O8P |
|---|
| SMILES | [H][C@@](COC(=O)CCCCCCCCCCCCCC)(COP(O)(O)=O)OC(=O)CCCCCCCCCCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-diacylglycerol-3-phosphates. These are glycerol-3-phosphates in which the glycerol moiety is bonded to two aliphatic chains through ester linkages. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerophospholipids |
|---|
| Sub Class | Glycerophosphates |
|---|
| Direct Parent | 1,2-diacylglycerol-3-phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-diacylglycerol-3-phosphate
- Fatty acid ester
- Monoalkyl phosphate
- Dicarboxylic acid or derivatives
- Fatty acyl
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Carboxylic acid ester
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Organooxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | - Cytoplasm
- Endoplasmic reticulum
- Mitochondrion membrane
|
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | | Cardiolipin Biosynthesis CL(15:0/16:0/15:0/16:0) | PW011737 |  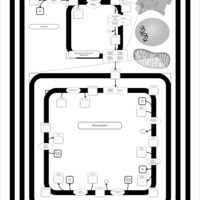  | | Cardiolipin Biosynthesis CL(15:0/16:0/16:0/23:1(11Z)) | PW011738 |    | | Cardiolipin Biosynthesis CL(15:0/16:0/16:0/23:1(9Z)) | PW011739 |    | | Cardiolipin Biosynthesis CL(15:0/16:0/16:0/25:0) | PW011740 |    | | Cardiolipin Biosynthesis CL(15:0/16:0/16:0/25:1(11Z)) | PW011741 |    |
|
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | |
|---|
| References |
|---|
| References: | - Rattray JB, Schibeci A, Kidby DK. (1975). "Lipids of yeasts." Bacteriol Rev. 1975 Sep;39(3):197-231.240350
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | FOODB ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|
