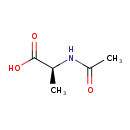
| GC-MS | GC-MS Spectrum - GC-MS (1 TMS) | splash10-05n0-2900000000-c0a5e66723f41ac78751 | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-05n0-2900000000-c0a5e66723f41ac78751 | JSpectraViewer | MoNA |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-82510327b6f9313f2dec | JSpectraViewer |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-9200000000-5c8085960ac907268dee | JSpectraViewer |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | JSpectraViewer |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | JSpectraViewer |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | JSpectraViewer |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0006-9000000000-4cea4517104b7a324deb | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0006-9000000000-4497242e6b422cf3f913 | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0006-9000000000-22d764f7b773b82c6515 | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-IT , negative | splash10-000i-9000000000-e6becb5f8b8bbb53304a | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9000000000-0aae7c100ca236c5d9ff | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-000i-9000000000-a834937166d1b13573af | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9000000000-64d13cbfedb6b9468f0f | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Negative | splash10-0079-9500000000-47ed2f6ecfc2c07a7238 | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9000000000-381b773ca35cc4fcd019 | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-5f8b9c01b2c31f02a57c | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-bb702a57406742ce92fd | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-0006-9000000000-bcbc1b15a23596134e9b | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9000000000-dcbe526c53d0cc355023 | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9000000000-9c03cb4f6a9e3f4d7d8d | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-7ab7ce4fbeeaa4906786 | JSpectraViewer | MoNA |
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-000i-9000000000-f747c686b81495308cfc | JSpectraViewer | MoNA |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0019-9600000000-98787fdcdf179af3efc6 | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000f-9000000000-c9b855453cf3c68830bf | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-4178e09fa261bbb5e7e9 | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-4900000000-2739a6e8536aca9442cc | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9200000000-2df5117d1f0498fa4d47 | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0076-9000000000-74175a91d6530fa275df | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000l-9100000000-5c3ebb626b607f2771cb | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-006x-9000000000-3526994d54c9aef41949 | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-527b4afd5857c451f463 | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available | JSpectraViewer |
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | JSpectraViewer |