| Identification |
|---|
| YMDB ID | YMDB01640 |
|---|
| Name | Astringin |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Brewer's yeast |
|---|
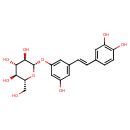
| Description | Astringin, also known as (e)-astringin, belongs to the class of organic compounds known as stilbene glycosides. Stilbene glycosides are compounds structurally characterized by the presence of a carbohydrate moiety glycosidically linked to the stilbene skeleton. Thus, astringin is considered to be an aromatic polyketide lipid molecule. Astringin is a very hydrophobic molecule, practically insoluble (in water), and relatively neutral. Astringin may be a unique S. cerevisiae (yeast) metabolite. |
|---|
| Structure | |
|---|
| Synonyms | - (e)-Astringin
- 3,4,3',5'-Tetrahydroxystilbene 3'-glucoside
- Piceatannol 3-b-D-glucoside
- Piceatannol 3-b-glucoside
- Piceatannol 3-beta-D-glucoside
- Piceatannol 3-beta-glucoside
- Piceatannol 3-O-b-D-glucoside
- Piceatannol 3-O-beta-D-glucoside
- Piceatannol 3-O-β-D-glucoside
- Piceatannol 3-β-D-glucoside
- Piceatannol 3-β-glucoside
- trans-Astringin
- 3-[(1E)-2-(3,4-Dihydroxyphenyl)ethenyl]-5-hydroxyphenyl beta-D-glucopyranoside
- 3-[(1E)-2-(3,4-Dihydroxyphenyl)ethenyl]-5-hydroxyphenyl β-D-glucopyranoside
- 3,4,3’,5’-Tetrahydroxystilbene 3’-glucoside
|
|---|
| CAS number | 29884-49-9 |
|---|
| Weight | Average: 406.3833
Monoisotopic: 406.126382302 |
|---|
| InChI Key | PERPNFLGJXUDDW-CUYWLFDKSA-N |
|---|
| InChI | InChI=1S/C20H22O9/c21-9-16-17(25)18(26)19(27)20(29-16)28-13-6-11(5-12(22)8-13)2-1-10-3-4-14(23)15(24)7-10/h1-8,16-27H,9H2/b2-1+/t16-,17-,18+,19-,20-/m1/s1 |
|---|
| IUPAC Name | (2S,3R,4S,5S,6R)-2-{3-[(E)-2-(3,4-dihydroxyphenyl)ethenyl]-5-hydroxyphenoxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| Traditional IUPAC Name | astringin |
|---|
| Chemical Formula | C20H22O9 |
|---|
| SMILES | OC[C@H]1O[C@@H](OC2=CC(O)=CC(\C=C\C3=CC=C(O)C(O)=C3)=C2)[C@H](O)[C@@H](O)[C@@H]1O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbene glycosides. Stilbene glycosides are compounds structurally characterized by the presence of a carbohydrate moiety glycosidically linked to the stilbene skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Stilbene glycosides |
|---|
| Direct Parent | Stilbene glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene glycoside
- Phenolic glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Phenoxy compound
- Phenol ether
- Catechol
- Styrene
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Oxane
- Monosaccharide
- Benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Hydrocarbon derivative
- Primary alcohol
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | Not Available |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | Not Available |
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 2899 | | HMDB ID | Not Available | | Pubchem Compound ID | 5089891 | | Kegg ID | C10245 | | ChemSpider ID | Not Available | | FOODB ID | Not Available | | Wikipedia ID | Astringin | | BioCyc ID | Not Available |
|
|---|