| Identification |
|---|
| YMDB ID | YMDB01609 |
|---|
| Name | 3-Oxo-beta-ionone |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Brewer's yeast |
|---|
| Description | 3-Oxo-beta-ionone belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. 3-Oxo-beta-ionone is an extremely weak basic (essentially neutral) compound (based on its pKa). |
|---|
| Structure | |
|---|
| Synonyms | - 2,4,4-trimethyl-3-(3-oxo-1-butenyl)cyclohex-2-en-1-one
- 2,4,4-Trimethyl-3-[(1E)-3-oxo-1-butenyl]-2-cyclohexen-1-one
- 3-Keto-beta-ionone
- 4-Oxo-beta-ionone
- 3-oxo-b-Ionone
- 3-oxo-Β-ionone
|
|---|
| CAS number | 27185-77-9 |
|---|
| Weight | Average: 206.2808
Monoisotopic: 206.13067982 |
|---|
| InChI Key | OBHGOXFSRVNKBS-UHFFFAOYSA-N |
|---|
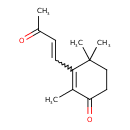
| InChI | InChI=1S/C13H18O2/c1-9(14)5-6-11-10(2)12(15)7-8-13(11,3)4/h5-6H,7-8H2,1-4H3 |
|---|
| IUPAC Name | 2,4,4-trimethyl-3-(3-oxobut-1-en-1-yl)cyclohex-2-en-1-one |
|---|
| Traditional IUPAC Name | 2,4,4-trimethyl-3-(3-oxobut-1-en-1-yl)cyclohex-2-en-1-one |
|---|
| Chemical Formula | C13H18O2 |
|---|
| SMILES | CC(=O)C=CC1=C(C)C(=O)CCC1(C)C |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Megastigmane sesquiterpenoid
- Cyclofarsesane sesquiterpenoid
- Ionone derivative
- Cyclohexenone
- Acryloyl-group
- Enone
- Alpha,beta-unsaturated ketone
- Ketone
- Cyclic ketone
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | Not Available |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | Not Available |
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ox-3900000000-e07e3214c50514fd81db | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0950000000-82de7bcbf5ca14d2b2f6 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052s-4910000000-b21c4dd1243c6c09c767 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066r-9500000000-00b17b7c231e59c0d0a2 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0290000000-d6b8b009d962e9f08f75 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-1790000000-5ebe9812818cbf1efc41 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-3900000000-e353efc723f307e518f3 | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Loscos, N., Hernandez-Orte, P., Cacho, J., Ferreira, V. (2007). "Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions." J Agric Food Chem 55:6674-6684.17616208
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 5363876 | | Kegg ID | Not Available | | ChemSpider ID | 21173162 | | FOODB ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|