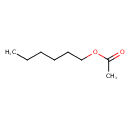
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9000000000-a501dc38f24dd902d772 | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9000000000-8bde2be982cc06c303c6 | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a5c-9000000000-6b68e54666d458c23e6b | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0ab9-9000000000-d6e7e57e88fb673af069 | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-9000000000-88601f99d038175f4279 | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9000000000-a501dc38f24dd902d772 | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-052f-9000000000-8bde2be982cc06c303c6 | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0a5c-9000000000-6b68e54666d458c23e6b | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0ab9-9000000000-d6e7e57e88fb673af069 | JSpectraViewer | MoNA |
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0006-9000000000-88601f99d038175f4279 | JSpectraViewer | MoNA |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-3491fb3e8a6ab76ee998 | JSpectraViewer |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-4900000000-28caa8bae540fff6cabf | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-9200000000-18516c816e1df27baa97 | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-1a25a9f1204ec52e74df | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-5900000000-c31f6fce2a7579cc03eb | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4l-9300000000-50527105286023147cca | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-898dec7fbf687dff6f20 | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-9000000000-14261d9db48ae9874c3a | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052f-9000000000-baac9328bd41e72b1fb6 | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-9000000000-3db04ec4ac853aef0df9 | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-1900000000-2ac8b7ac46df462cdaaa | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-c01bbbf5bed889264ddb | JSpectraViewer |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-3e44aaba3cc3df109037 | JSpectraViewer |
| MS | Mass Spectrum (Electron Ionization) | splash10-052f-9000000000-f72b4cda7606150aa804 | JSpectraViewer | MoNA |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
| 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer |