| Identification |
|---|
| YMDB ID | YMDB01124 |
|---|
| Name | DG(14:0/16:0/0:0) |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Brewer's yeast |
|---|
| Description | DG(14:0/16:0/0:0) belongs to the family of Diacylglycerols. These are glycerolipids lipids containing a common glycerol backbone to which at least one fatty acyl group is esterified. DG(14:0/16:0/0:0) is also a substrate of diacylglycerol kinase. It is involved in the phospholipid metabolic pathway. |
|---|
| Structure | |
|---|
| Synonyms | - 1-myristoyl-2-palmitoyl-sn-glycerol
- DAG(14:0/16:0)
- DAG(30:0)
- DG(14:0/16:0)
- DG(30:0)
- Diacylglycerol
- Diacylglycerol(14:0/16:0)
- Diacylglycerol(30:0)
- Diglyceride
|
|---|
| CAS number | Not Available |
|---|
| Weight | Average: 540.87
Monoisotopic: 540.475375161 |
|---|
| InChI Key | NYNQTTOUQMCOEM-WJOKGBTCSA-N |
|---|
| InChI | InChI=1S/C33H64O5/c1-3-5-7-9-11-13-15-16-18-20-22-24-26-28-33(36)38-31(29-34)30-37-32(35)27-25-23-21-19-17-14-12-10-8-6-4-2/h31,34H,3-30H2,1-2H3/t31-/m1/s1 |
|---|
| IUPAC Name | (2R)-1-hydroxy-3-(tetradecanoyloxy)propan-2-yl hexadecanoate |
|---|
| Traditional IUPAC Name | (2R)-1-hydroxy-3-(tetradecanoyloxy)propan-2-yl hexadecanoate |
|---|
| Chemical Formula | C33H64O5 |
|---|
| SMILES | [H][C@@](CO)(COC(=O)CCCCCCCCCCCCC)OC(=O)CCCCCCCCCCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-diacylglycerols. These are diacylglycerols containing a glycerol acylated at positions 1 and 2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Diradylglycerols |
|---|
| Direct Parent | 1,2-diacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,2-acyl-sn-glycerol
- Fatty acid ester
- Fatty acyl
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | Not Available |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | | Triacylglycerol metabolism TG(14:0/14:0/16:0) | PW007653 |   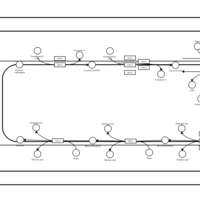 | | Triacylglycerol metabolism TG(14:0/14:1(9Z)/16:0) | PW007666 |   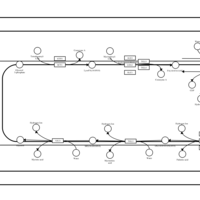 | | Triacylglycerol metabolism TG(14:0/15:0/16:0) | PW007680 |  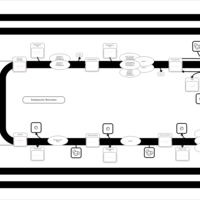 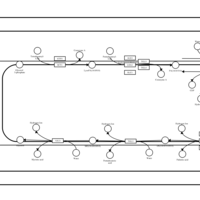 | | Triacylglycerol metabolism TG(14:0/16:0/16:0) | PW007709 | 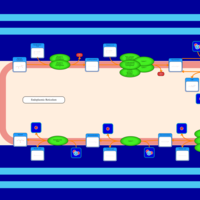 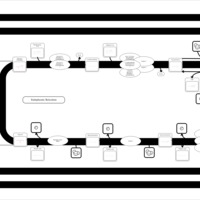 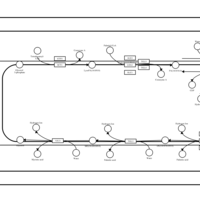 | | Triacylglycerol metabolism TG(14:0/16:0/25:0) | PW007854 |  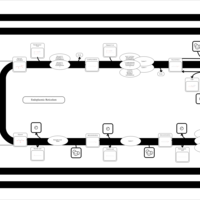  |
|
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | | Intracellular Concentration | Substrate | Growth Conditions | Strain | Citation |
|---|
| 8750 ± 2975 umol/L | SD media with 2% raffinose | 24 oC | BY4741 | PMID: 19174513 | | 3250 ± 875 umol/L | SD media with 2% raffinose | 37 oC | BY4741 | PMID: 19174513 | | Conversion Details Here |
|
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01p9-0094030000-dec2423206eb7cf70ee2 | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Ejsing, C. S., Sampaio, J. L., Surendranath, V., Duchoslav, E., Ekroos, K., Klemm, R. W., Simons, K., Shevchenko, A. (2009). "Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry." Proc Natl Acad Sci U S A 106:2136-2141.19174513
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB07011 | | Pubchem Compound ID | 4577178 | | Kegg ID | C00165 | | ChemSpider ID | 24765845 | | FOODB ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|
