| Identification |
|---|
| YMDB ID | YMDB00516 |
|---|
| Name | 5-Formyltetrahydrofolic acid |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Baker's yeast |
|---|
| Description | 5-Formyltetrahydrofolate (N5-Formyl-THF) is a folate coenzyme. Tetrahydrofolate (vitamin B9) and its derivatives, commonly termed folates, are essential cofactors that facilitate the transfer of one-carbon units from donor molecules into important biosynthetic pathways leading to methionine, purine, and pyrimidine biosynthesis. Folates also mediate the interconversion of serine and glycine, and play a role in histidine catabolism. Although 5-Formyl-tetrahydrofolate is the most stable derivative of the reduced folates, it is the only folate derivative that does not serve as a cofactor in C1-metabolism. [Biocyc PWY-2201] [PMID: 11923304] |
|---|
| Structure | |
|---|
| Synonyms | - (6R,S)-5-Formyltetrahydrofolate
- (6S)-5-formyltetrahydrofolate
- (6S)-5-HCO-H4folate
- [(6S)-5-formyl-5,6,7,8-tetrahydropteroyl]glutamate
- 10-Formyl-7,8-dihydrofolate
- 10-Formyl-7,8-dihydrofolic acid
- 5-Formyl-5,6,7,8-tetrahydrofolate
- 5-Formyl-5,6,7,8-tetrahydrofolic acid
- 5-Formyltetrahydrofolate
- 5-formyltetrahydrofolic acid
- 5-Formyltetrahydropteroylglutamate
- 5-Formyltetrahydropteroylglutamic acid
- folinate
- folinic acid
- Folinic acid-SF
- l-Leucovorin
- l(-)-5-formyl-5,6,7,8-tetrahydrofolic acid
- Leucal
- Leucovorin
- Levoleucovorin
- N-(5-formyl-5,6,7,8-tetrahydropteroyl)-L-glutamic acid
- N5-Formyl-5,6,7,8-tetrahydrofolate
- N5-Formyl-5,6,7,8-tetrahydrofolic acid
- N5-Formyltetrahydrofolate
- N5-Formyltetrahydrofolic acid
- Welcovorin
- (6S)-5-Formyl-5,6,7,8-tetrahydrofolic acid
- (6S)-Folinic acid
- (6S)-Leucovorin
- (S)-Leucovorin
- Citrovorum factor
- L-Folinic acid
- Levofolene
- Levofolinic acid
- N-[4-({[(6S)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)benzoyl]-L-glutamic acid
- (6S)-5-Formyl-5,6,7,8-tetrahydrofolate
- (6S)-Folinate
- L-Folinate
- Levofolinate
- N-[4-({[(6S)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)benzoyl]-L-glutamate
- Pteroyl-D-glutamate
- 5 Formyltetrahydropteroylglutamate
- Calcium folinate
- Leucovorin, (S)-isomer
- Leucovorin, monosodium salt
- Leucovorin, (R)-isomer
- Leucovorin, calcium
- Leucovorin, calcium (1:1) salt, (DL)-isomer
- Leucovorin, calcium (1:1) salt, (S)-isomer
- Leukovorin
- Leukovorum
- Monosodium salt leucovorin
- Wellcovorin
- Levo leucovorin
- 6 S Leucovorin
- Acid, folinic
- Factor, citrovorum
- Folinic acid SF
- Fusilev
- Leucovorin, calcium (1:1) salt, pentahydrate
- Leucovorin, monopotassium salt, (S)-isomer
- N(5)-Formyltetrahydrofolate
- S-Leucovorin
- 5 Formyltetrahydrofolate
- 6-S-Leucovorin
- 6S Leucovorin
- 6S-Leucovorin
- Calcium leucovorin
- Folinate, calcium
- Leucovorin, (D)-isomer
- Leucovorin, (DL)-isomer
- Leucovorin, calcium (1:1) salt
- Leucovorin, monosodium salt, (S)-isomer
- S Leucovorin
- Levo-leucovorin
- 5-Formyl-(6S)-tetrahydrofolic acid
- CF
- Kunyrin
- Leucoverin
- N5-Formyl-THF
- (6S)-5-Formyltetrahydrofolic acid
|
|---|
| CAS number | 58-05-9 |
|---|
| Weight | Average: 473.4393
Monoisotopic: 473.165896125 |
|---|
| InChI Key | VVIAGPKUTFNRDU-STQMWFEESA-N |
|---|
| InChI | InChI=1S/C20H23N7O7/c21-20-25-16-15(18(32)26-20)27(9-28)12(8-23-16)7-22-11-3-1-10(2-4-11)17(31)24-13(19(33)34)5-6-14(29)30/h1-4,9,12-13,22H,5-8H2,(H,24,31)(H,29,30)(H,33,34)(H4,21,23,25,26,32)/t12-,13-/m0/s1 |
|---|
| IUPAC Name | (2S)-2-{[4-({[(6S)-2-amino-5-formyl-4-oxo-1,4,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)phenyl]formamido}pentanedioic acid |
|---|
| Traditional IUPAC Name | (2S)-2-{[4-({[(6S)-2-amino-5-formyl-4-oxo-1,6,7,8-tetrahydropteridin-6-yl]methyl}amino)phenyl]formamido}pentanedioic acid |
|---|
| Chemical Formula | C20H23N7O7 |
|---|
| SMILES | [H]C(=O)N1[C@@H](CNC2=CC=C(C=C2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)CNC2=C1C(=O)NC(N)=N2 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydrofolic acids. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Tetrahydrofolic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydrofolic acid
- Glutamic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Hippuric acid
- Hippuric acid or derivatives
- Alpha-amino acid or derivatives
- Aminobenzamide
- Aminobenzoic acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Benzoyl
- Aniline or substituted anilines
- Phenylalkylamine
- Aminopyrimidine
- Secondary aliphatic/aromatic amine
- Pyrimidone
- Dicarboxylic acid or derivatives
- Benzenoid
- Pyrimidine
- Monocyclic benzene moiety
- Heteroaromatic compound
- Vinylogous amide
- Tertiary carboxylic acid amide
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Secondary amine
- Carboxylic acid
- Carboxylic acid derivative
- Azacycle
- Organooxygen compound
- Carbonyl group
- Organic nitrogen compound
- Hydrocarbon derivative
- Amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | 245 °C |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | Not Available |
|---|
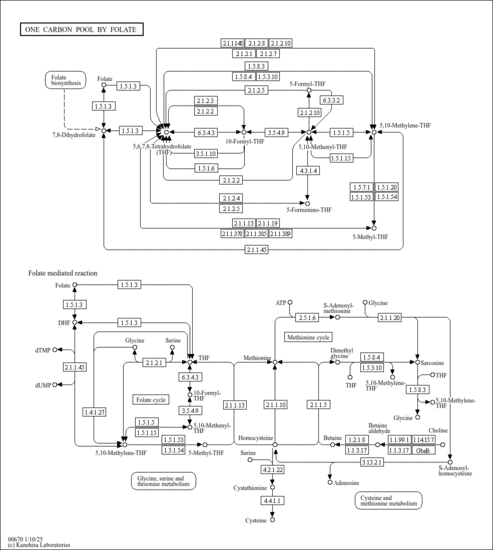
| KEGG Pathways | |
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0adi-0031900000-15cbae867ecd4a003ef0 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0393400000-73f11f6c59198eafb45f | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pb9-0591000000-ea5c6303d77ffa4af0cb | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-a3ae54a4d9b2cae45b72 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00bc-1243900000-4ea7b3b1902e6a1204e5 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-7973000000-df3edd5bd8661611dd06 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00fr-0005900000-5985bdc6c069ecc00046 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-056s-1597400000-db859aadd61942f2e80b | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05o0-0921000000-ddf359b6fc3776d45401 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000900000-56bf354a786254d088f4 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fb9-1220900000-d93259006e38248d26b4 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f6x-5920100000-575460bfef119781af80 | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Scheer, M., Grote, A., Chang, A., Schomburg, I., Munaretto, C., Rother, M., Sohngen, C., Stelzer, M., Thiele, J., Schomburg, D. (2011). "BRENDA, the enzyme information system in 2011." Nucleic Acids Res 39:D670-D676.21062828
- Herrgard, M. J., Swainston, N., Dobson, P., Dunn, W. B., Arga, K. Y., Arvas, M., Bluthgen, N., Borger, S., Costenoble, R., Heinemann, M., Hucka, M., Le Novere, N., Li, P., Liebermeister, W., Mo, M. L., Oliveira, A. P., Petranovic, D., Pettifer, S., Simeonidis, E., Smallbone, K., Spasic, I., Weichart, D., Brent, R., Broomhead, D. S., Westerhoff, H. V., Kirdar, B., Penttila, M., Klipp, E., Palsson, B. O., Sauer, U., Oliver, S. G., Mendes, P., Nielsen, J., Kell, D. B. (2008). "A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology." Nat Biotechnol 26:1155-1160.18846089
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | |
|---|