| Identification |
|---|
| YMDB ID | YMDB00466 |
|---|
| Name | trans-dodec-2-enoyl-CoA |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Baker's yeast |
|---|
| Description | (2E)-Dodecenoyl-CoA, also known as (2E)-dodec-2-enoyl-CoA or 2-trans-dodecenoyl-CoA, belongs to the class of organic compounds known as medium-chain 2-enoyl coas. These are organic compounds containing a coenzyme A substructure linked to a medium-chain 2-enoyl chain of 5 to 12 carbon atoms. Based on a literature review very few articles have been published on (2E)-Dodecenoyl-CoA. |
|---|
| Structure | |
|---|
| Synonyms | - (2E)-Dodec-2-enoyl-CoA
- (2E)-Dodec-2-enoyl-Coenzyme A
- (2E)-Dodecenoyl-CoA
- 2-trans-dodecenoyl-coa
- 2-trans-Dodecenoyl-Coenzyme A
|
|---|
| CAS number | 1066-12-2 |
|---|
| Weight | Average: 947.821
Monoisotopic: 947.266623627 |
|---|
| InChI Key | IRFYVBULXZMEDE-DEEZISNZSA-N |
|---|
| InChI | InChI=1S/C33H56N7O17P3S/c1-4-5-6-7-8-9-10-11-12-13-24(42)61-17-16-35-23(41)14-15-36-31(45)28(44)33(2,3)19-54-60(51,52)57-59(49,50)53-18-22-27(56-58(46,47)48)26(43)32(55-22)40-21-39-25-29(34)37-20-38-30(25)40/h12-13,20-22,26-28,32,43-44H,4-11,14-19H2,1-3H3,(H,35,41)(H,36,45)(H,49,50)(H,51,52)(H2,34,37,38)(H2,46,47,48)/b13-12+/t22-,26-,27-,28+,32-/m1/s1 |
|---|
| IUPAC Name | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-2-({[({[(3R)-3-{[2-({2-[(2E)-dodec-2-enoylsulfanyl]ethyl}carbamoyl)ethyl]carbamoyl}-3-hydroxy-2,2-dimethylpropoxy](hydroxy)phosphoryl}oxy)(hydroxy)phosphoryl]oxy}methyl)-4-hydroxyoxolan-3-yl]oxy}phosphonic acid |
|---|
| Traditional IUPAC Name | trans-dodec-2-enoyl-coa |
|---|
| Chemical Formula | C33H56N7O17P3S |
|---|
| SMILES | CCCCCCCCC\C=C\C(=O)SCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP(=O)(O)OP(=O)(O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain 2-enoyl coas. These are organic compounds containing a coenzyme A substructure linked to a medium-chain 2-enoyl chain of 5 to 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
| Direct Parent | Medium-chain 2-enoyl CoAs |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coenzyme a or derivatives
- Purine ribonucleoside 3',5'-bisphosphate
- Purine ribonucleoside bisphosphate
- Purine ribonucleoside diphosphate
- Ribonucleoside 3'-phosphate
- Pentose phosphate
- Pentose-5-phosphate
- Beta amino acid or derivatives
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- N-acyl-amine
- N-substituted imidazole
- Organic phosphoric acid derivative
- Monosaccharide
- Pyrimidine
- Alkyl phosphate
- Fatty amide
- Phosphoric acid ester
- Tetrahydrofuran
- Imidazole
- Azole
- Heteroaromatic compound
- Carbothioic s-ester
- Secondary alcohol
- Thiocarboxylic acid ester
- Carboxamide group
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Sulfenyl compound
- Thiocarboxylic acid or derivatives
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Organosulfur compound
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic nitrogen compound
- Primary amine
- Organopnictogen compound
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | - Endoplasmic Reticulum, Lipid Particle, Peroxisome
|
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | |
|---|
| KEGG Pathways | | Fatty acid elongation in mitochondria | ec00062 | 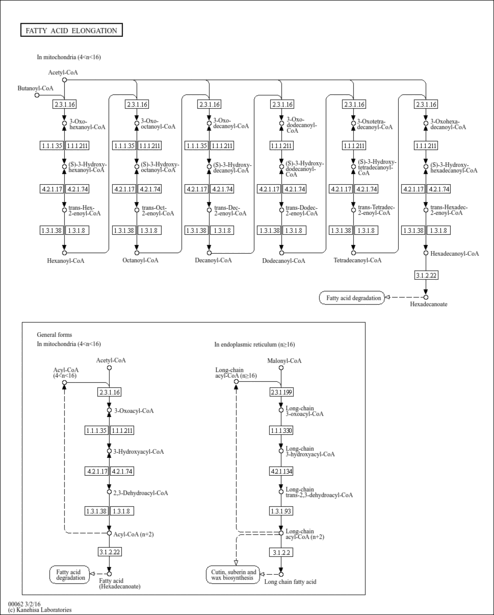 | | Fatty acid metabolism | ec00071 | 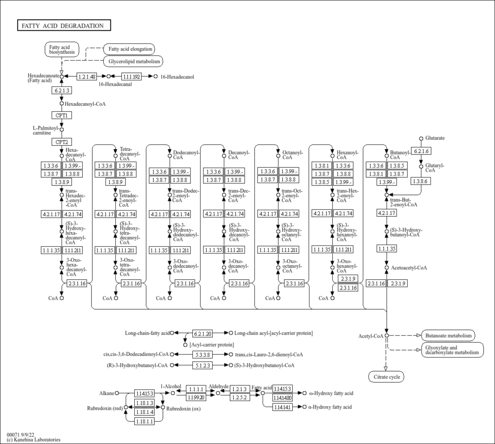 |
|
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1945000202-3935b90e93f42c57c383 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0963100000-76e7d2ecf42a69de257f | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1931000000-0a31c1caf454ec14312b | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0059-3911032304-f163cfb1d8b6995c8f24 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-003r-2900110001-7ef123400b090afe0b26 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-7900000000-349d97519ce451369a61 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0000000019-58bf1dfa7c94908bff4f | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1901000456-82b2f81fe92b050b4cdb | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-0001900000-07c324a5e8565c914e97 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000000009-983824b3dc4935215fda | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-4200302409-0c27a425d05507057591 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00p0-3302501906-4e51e92e28749536f9b4 | JSpectraViewer | | MS | Mass Spectrum (Electron Ionization) | Not Available | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Herrgard, M. J., Swainston, N., Dobson, P., Dunn, W. B., Arga, K. Y., Arvas, M., Bluthgen, N., Borger, S., Costenoble, R., Heinemann, M., Hucka, M., Le Novere, N., Li, P., Liebermeister, W., Mo, M. L., Oliveira, A. P., Petranovic, D., Pettifer, S., Simeonidis, E., Smallbone, K., Spasic, I., Weichart, D., Brent, R., Broomhead, D. S., Westerhoff, H. V., Kirdar, B., Penttila, M., Klipp, E., Palsson, B. O., Sauer, U., Oliver, S. G., Mendes, P., Nielsen, J., Kell, D. B. (2008). "A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology." Nat Biotechnol 26:1155-1160.18846089
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | |
|---|


