| Identification |
|---|
| YMDB ID | YMDB00363 |
|---|
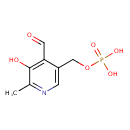
| Name | Pyridoxal 5'-phosphate |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Baker's yeast |
|---|
| Description | Pyridoxal 5'-phosphate, also known as phosphopyridoxal or PLP, belongs to the class of organic compounds known as pyridoxals and derivatives. Pyridoxals and derivatives are compounds containing a pyridoxal moiety, which consists of a pyridine ring substituted at positions 2,3,4, and 5 by a methyl group, a hydroxyl group, a carbaldehyde group, and a hydroxymethyl group, respectively. Pyridoxal 5'-phosphate is a strong basic compound (based on its pKa). Pyridoxal 5'-phosphate exists in all living species, ranging from bacteria to humans. Pyridoxal 5'-phosphate is a potentially toxic compound. |
|---|
| Structure | |
|---|
| Synonyms | - Apolon B6
- Biosechs
- Codecarboxylase
- Coenzyme B6
- Hairoxal
- Hexermin-P
- Hi-Pyridoxin
- Hiadelon
- Himitan
- PAL-P
- Phosphopyridoxal
- Phosphopyridoxal coenzyme
- Pidopidon
- Piodel
- PLP
- Pydoxal
- Pyridoxal 5-phosphate
- Pyridoxal 5'-phosphate
- Pyridoxal 5'-phosphic acid
- Pyridoxal P
- Pyridoxal phosphate
- Pyridoxal-P
- Pyridoxyl phosphate
- Pyromijin
- Sechvitan
- Vitahexin-P
- Vitazechs
- 3-Hydroxy-2-methyl-5-[(phosphonooxy)methyl]-4-pyridinecarboxaldehyde
- 3-Hydroxy-5-(hydroxymethyl)-2-methylisonicotinaldehyde 5-phosphate
- Phosphoric acid mono-(4-formyl-5-hydroxy-6-methyl-pyridin-3-ylmethyl) ester
- Pyridoxal 5'-(dihydrogen phosphate)
- Pyridoxal 5-monophosphoric acid ester
- PYRIDOXAL-5'-phosphATE
- 3-Hydroxy-5-(hydroxymethyl)-2-methylisonicotinaldehyde 5-phosphoric acid
- Phosphate mono-(4-formyl-5-hydroxy-6-methyl-pyridin-3-ylmethyl) ester
- Pyridoxal 5'-(dihydrogen phosphoric acid)
- Pyridoxal 5-monophosphate ester
- Pyridoxal 5-phosphoric acid
- Pyridoxal phosphoric acid
- PYRIDOXAL-5'-phosphoric acid
- Pyridoxal 5'-phosphoric acid
- Phosphate, pyridoxal
- Pyridoxal 5 phosphate
|
|---|
| CAS number | 54-47-7 |
|---|
| Weight | Average: 247.1419
Monoisotopic: 247.024573569 |
|---|
| InChI Key | NGVDGCNFYWLIFO-UHFFFAOYSA-N |
|---|
| InChI | InChI=1S/C8H10NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2-3,11H,4H2,1H3,(H2,12,13,14) |
|---|
| IUPAC Name | [(4-formyl-5-hydroxy-6-methylpyridin-3-yl)methoxy]phosphonic acid |
|---|
| Traditional IUPAC Name | pyridoxal phosphate |
|---|
| Chemical Formula | C8H10NO6P |
|---|
| SMILES | [H]OC1=C(C([H])=O)C(=C([H])N=C1C([H])([H])[H])C([H])([H])OP(=O)(O[H])O[H] |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridoxals and derivatives. Pyridoxals and derivatives are compounds containing a pyridoxal moiety, which consists of a pyridine ring substituted at positions 2,3,4, and 5 by a methyl group, a hydroxyl group, a carbaldehyde group, and a hydroxymethyl group, respectively. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridine carboxaldehydes |
|---|
| Direct Parent | Pyridoxals and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridoxal
- Aryl-aldehyde
- Monoalkyl phosphate
- Hydroxypyridine
- Methylpyridine
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Vinylogous acid
- Heteroaromatic compound
- Azacycle
- Organopnictogen compound
- Aldehyde
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Solid |
|---|
| Charge | 0 |
|---|
| Melting point | 255 °C |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | 28 mg/mL [HMP experimental] | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | |
|---|
| Organoleptic Properties | Not Available |
|---|
| SMPDB Pathways | |
|---|
| KEGG Pathways | |
|---|
| SMPDB Reactions | |
|---|
| KEGG Reactions | |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 3 TMS) | splash10-0gb9-2690000000-c9bacb7e657461e28407 | JSpectraViewer | MoNA | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0gb9-2690000000-c9bacb7e657461e28407 | JSpectraViewer | MoNA | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-004j-0910000000-0408f3957221fc9882cd | JSpectraViewer | MoNA | | GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0gb9-1790000000-b01115fa8b4cfd3612c7 | JSpectraViewer | MoNA | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-9520000000-bfd574b2e5355694902a | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-006t-9152000000-26c9f631195663322986 | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0f6t-0690000000-4aa57f3f28f0bdef9dcf | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0fxx-8900000000-ee972556340c61650528 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-014l-9100000000-3cc29fb51820adecd59c | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0002-0090000000-9b86e80a9dd0de14ab59 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-03dj-9800000000-9313aa9cbf19bc6311c5 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0002-9500000000-7ce120a58879e2214ce5 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-004j-9200000000-7e6bd8c298613e59fb5d | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-004i-9000000000-4c05178b1645bf01f3fa | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0udi-3920000000-6c5e6106f5658d1d6c50 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-0udi-3920000000-f96097815e6922356131 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-002b-9000000000-2c4e243699a95e3a0f88 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-002b-9000000000-8aa111485891af9f0d02 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0002-0090000000-9b86e80a9dd0de14ab59 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-03dj-9800000000-9313aa9cbf19bc6311c5 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0002-9500000000-7ce120a58879e2214ce5 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004j-9200000000-7e6bd8c298613e59fb5d | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004i-9000000000-4c05178b1645bf01f3fa | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-002b-9000000000-2c4e243699a95e3a0f88 | JSpectraViewer | MoNA | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , negative | splash10-002b-9000000000-8aa111485891af9f0d02 | JSpectraViewer | MoNA | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f6t-1790000000-1dcb07cd1110c71f7005 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0900000000-ca6aab7f31a36863a417 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9800000000-053802b9c0533d7943ea | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-9080000000-12ca63d34d39d59f6b42 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-144099ec201adbbc4684 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-70a9559d65e78c488e7e | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available | JSpectraViewer | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - UniProt Consortium (2011). "Ongoing and future developments at the Universal Protein Resource." Nucleic Acids Res 39:D214-D219.21051339
- Scheer, M., Grote, A., Chang, A., Schomburg, I., Munaretto, C., Rother, M., Sohngen, C., Stelzer, M., Thiele, J., Schomburg, D. (2011). "BRENDA, the enzyme information system in 2011." Nucleic Acids Res 39:D670-D676.21062828
- Herrgard, M. J., Swainston, N., Dobson, P., Dunn, W. B., Arga, K. Y., Arvas, M., Bluthgen, N., Borger, S., Costenoble, R., Heinemann, M., Hucka, M., Le Novere, N., Li, P., Liebermeister, W., Mo, M. L., Oliveira, A. P., Petranovic, D., Pettifer, S., Simeonidis, E., Smallbone, K., Spasic, I., Weichart, D., Brent, R., Broomhead, D. S., Westerhoff, H. V., Kirdar, B., Penttila, M., Klipp, E., Palsson, B. O., Sauer, U., Oliver, S. G., Mendes, P., Nielsen, J., Kell, D. B. (2008). "A consensus yeast metabolic network reconstruction obtained from a community approach to systems biology." Nat Biotechnol 26:1155-1160.18846089
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | |
|---|