| Identification |
|---|
| YMDB ID | YMDB01451 |
|---|
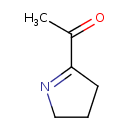
| Name | 2-acetyl-1-pyrroline |
|---|
| Species | Saccharomyces cerevisiae |
|---|
| Strain | Brewer's yeast |
|---|
| Description | 2-Acetyl-1-pyrroline, abbreviated 2AP is an aroma compound and flavor that gives white bread, jasmine rice and basmati rice, the spice pandan (Pandanus amaryllifolius), and bread flowers (Vallaris glabra) their typical smell. 2-Acetyl-1-pyrroline and its structural homolog, 6-acetyl-2,3,4,5-tetrahydropyridine of similar smell, can be formed by Maillard reactions during heating of food such as in baked bread. Both compounds have odor thresholds below 0.06 ng/l.[Wikipedia] |
|---|
| Structure | |
|---|
| Synonyms | - 1-(3,4-Dihydro-2H-pyrrol-5-yl)ethanone
- 1-Pyrroline, 2-acetyl
- 2-Acetyl-1-pyrolline
- 2-Acetyl-4,5-dihydro-3H-pyrrole
- 2AP
- APR
- 1-(3,4-dihydro-2H-Pyrrol-5-yl)ethanone, 9ci
- 2-Acetyl-1-pyrroline
- 2-Acetylpyrroline
|
|---|
| CAS number | 99583-29-6 |
|---|
| Weight | Average: 111.1418
Monoisotopic: 111.068413915 |
|---|
| InChI Key | DQBQWWSFRPLIAX-UHFFFAOYSA-N |
|---|
| InChI | InChI=1S/C6H9NO/c1-5(8)6-3-2-4-7-6/h2-4H2,1H3 |
|---|
| IUPAC Name | 1-(3,4-dihydro-2H-pyrrol-5-yl)ethan-1-one |
|---|
| Traditional IUPAC Name | 2-acetyl-1-pyrroline |
|---|
| Chemical Formula | C6H9NO |
|---|
| SMILES | [H]C([H])([H])C(=O)C1=NC([H])([H])C([H])([H])C1([H])[H] |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrrolines. Pyrrolines are compounds containing a pyrroline ring, which is a five-member unsaturated aliphatic ring with one nitrogen atom and four carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrrolines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Pyrrolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrroline
- Ketimine
- Ketone
- Propargyl-type 1,3-dipolar organic compound
- Organic 1,3-dipolar compound
- Azacycle
- Carbonyl group
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Imine
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Charge | 0 |
|---|
| Melting point | Not Available |
|---|
| Experimental Properties | | Property | Value | Reference |
|---|
| Water Solubility | Not Available | PhysProp | | LogP | Not Available | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations | Not Available |
|---|
| Organoleptic Properties | |
|---|
| SMPDB Pathways | Not Available |
|---|
| KEGG Pathways | Not Available |
|---|
| SMPDB Reactions | Not Available |
|---|
| KEGG Reactions | Not Available |
|---|
| Concentrations |
|---|
| Intracellular Concentrations | Not Available |
|---|
| Extracellular Concentrations | Not Available |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-014l-9000000000-b2ec2a5a737593af1876 | JSpectraViewer | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-5900000000-ba7d8b3068bce1b47b39 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ox-9300000000-d40b8a0858de158c3dd1 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9000000000-93e30e06cf0f3fac2e9e | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1900000000-c99599e8eef94b8c0068 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-3900000000-24d2c60642b551195027 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-a6046c2688bcfbb4c313 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1900000000-24735682b0a5560e64ae | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-9500000000-2e635455b88717ca2f97 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-a0c8a0fe2acbc430c438 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-3900000000-7cddab0b1712fe3943e6 | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014l-9100000000-a2ea5be954e40e1ce67b | JSpectraViewer | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9000000000-efc17d3cfd9d54e8a0c4 | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 1H NMR Spectrum | Not Available | JSpectraViewer | | 1D NMR | 13C NMR Spectrum | Not Available | JSpectraViewer |
|
|---|
| References |
|---|
| References: | - Mahadevan, K., Farmer, L. (2006). "Key odor impact compounds in three yeast extract pastes." J Agric Food Chem 54:7242-7250.16968089
|
|---|
| Synthesis Reference: | Not Available |
|---|
| External Links: | |
|---|